Physico-Chemical Study of the Adsorption of Carotenoids from Carrots on Raw and Modified Kaolinites ()
1. Introduction
Carotenoids are hydrocarbon compounds mainly synthesized by plants. They are responsible for the intensity of the yellow, orange and red colors of many plants and vegetables, their color is the result of the presence of a system of conjugated double bonds in the molecule. Carrots are another great source of carotenoids. In 2007, annual production was 20.601.214 tons [1] of which 33% was grown in China. Carrot cultivation is gaining momentum in Cameroon [2] . This crop is one of the most cultivated in peri-urban areas. Carrots are very perishable; however, it is observed that carrot extracts are used in beauty products [3] [4] .
Indeed, solar radiation interacts with the substances contained in the air to produce singlet oxygen and other oxygenated species. These species can attack the skin. Carotenoids, when present in cosmetic formulations, are likely to react with oxidizing species. it is also necessary to determine the real oxidizing power of carotenoids. Cyclic voltammetry is the most widely used and also the most effective method for determining the antioxidant power of chemical substances [5] [6] [7] [8] [9] .
Carotenoids have double bonds that give them significant antioxidant power. This antioxidant power can be particularly valorized in beauty masks in cosmetics [10] [11] [12] . Carotenoids are very useful in dermatology to improve the general condition of the skin, they promote the synthesis of melanin, which is the pigment responsible for tanning, and the natural filter of ultraviolet rays [13] ; they attenuate the formation of peroxide radicals [14] [15] facilitate the renewal of cutaneous tissues, attenuate scars and stretch marks and prevent the oxidation of lipids [16] [17] [18] [19] . The antioxidant properties of carotenoids allow them to trap reactive oxidant particles in cell tissue responsible for premature skin aging, expression lines, dry and damaged skin [20] . However, they are quickly destroyed by solar radiation; for this purpose, the use through a clay support as a beauty mask will allow a slow and deep diffusion in the skin, which would increase their concentration and consequently increase their protective action of the skin.
In addition, Cameroon has many clay deposits. In general, smectite-type clays are found in the North and Far North regions of Cameroon and, on the other hand, in southern Cameroon, kaolinite-type clays are found [21] [22] . The adsorbent properties of clays can be used for this purpose; moreover, natural products play a critical role in cosmetics, giving it an image of safety and relief compared to synthetic products, especially in anti-aging products [23] .
2. Material and Methods
2.1. Raw Materials
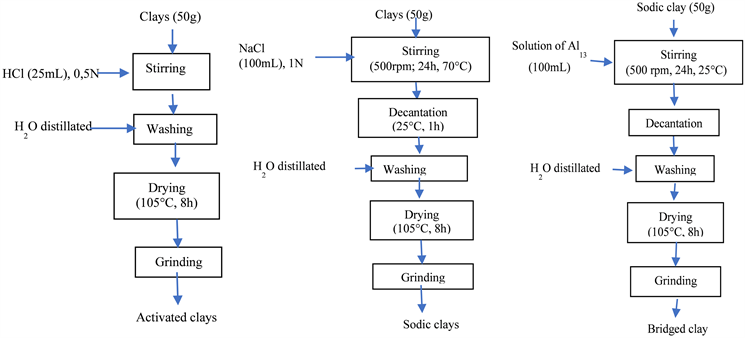
The carotenoid source consists of carrots (genus Dauca of Umbelliferae, species Dauca carota). They were harvested when ripe, sorted, washed several times with water; peeled and chopped using a stainless-steel scraper. We obtain fine fibers of one millimeter in diameter and about 5mm in length. These carrot fibers are then spread out on plastic racks and placed in an electric dryer (massa dry) at 40˚C for 24 hours. After drying, these fibers are stored in a black polyethylene bag. The clays were sampled in Douala in the littoral region and characterized by [24] and codified D0M for the raw, D05M for the clay activated with HCl 0.5N and DAl13 for the sample bridged with Aluminum Chloride (illustrated on the schemes below).
2.2. Equipments
The characterization of the carotenoids was carried out by UV-Visible spectrophotometry using a Perkin Elmer brand spectrophotometer (lambda 35) controlled by a computer, thanks to the Winlab Software program. The spectra were obtained by automatic scanning between 190 nm and 1100 nm. Quartz cells with a 1 cm optical path were used for this purpose.
The antioxidant activity of the carotenoids was determined by cyclic voltammetry, using a Bio-Logic SAS, system of three conventional electrodes at room temperature (25˚C) in the CH2Cl2 solution. For electrochemical measurements, a platinum electrode is considered as the working electrode, the platinum wire is the auxiliary electrode and the calomel saturated electrode is the reference electrode. Cyclic voltammograms are recorded from ±0.1 V to ±2.0 V; the speeds vary from 2 to 100 mV/S. The sample solution of pure β carotene is added to the experimental electrolyte in the electrochemical cell. The solution prepared is of low concentration (10−6 mM). Voltammograms are recorded immediately after the electrodes are immersed in the solution.
2.3. Technics Analysis
The β carotene solutions serving as reference are prepared from a standard solution obtained near the company Sigma-Aldrich (M) Pte. ltd. of 98% purity. Whose physicochemical characterizations and chemical structure are shown in Figure 1 and in Table 1.
The calibration curve is determined by taking 2.5 mg of β carotene powder which is introduced into 50 ml of cyclohexane. Necessary dilutions make it possible to obtain solutions of 10, 20, 30, 40 and 50 mg/L of β carotenes. Thus, the β-carotene content can be calculated with respect to the equation of the calibration curve.
For studies of adsorption of carotenoids by clays, we have in a series of 100 ml Erlenmeyer flasks covered with aluminum foil, introduced masses (2 g) of
![]()
Figure 1. Molecular structure of β carotene.
![]()
Table 1. Some characteristics of β carotene.
adsorbent to which we have added the same volume (5 ml) of carotenoid solution of variable concentration (0 to 50 mg/l). These closed bottles are placed on magnetic stirrers (IKA® RCT basie safety control model) with a regulated stirring speed of 250 rpm for a predetermined contact time. At the end of this time, the contents of the flask are centrifuged at 1500 revolutions/minute, and analyzed by UV-Visible spectrophotometry at 450 nm. Each experimental value is the average of two trials. The number of carotenoids per grams is given by the following relationship (Equation (1)):
(1)
where: qt is the quantity of carotenoids per unit mass of adsorbent (in mg/g);
C0 is the initial concentration of carotenoids (mg/L);
Ct is the residual concentration at time t (mg/L);
V is the volume of adsorbate (L);
m is the mass of adsorbent (g).
Desorption is the opposite phenomena of adsorption, by which the adsorbed molecules are detached from the substrate. Generally endothermic operation. The desorption studies were carried out using the method [25] modified. Indeed, after carrying out the adsorption, the clay on which the carotenoid is fixed is recovered, the solvent is introduced and kinetics are carried out in order to determine the time after which the maximum number of carotenoids has entered the solvent.
3. Results and Discussion
3.1. Characterization of β-Carotene
Figure 2 shows the determination of the maximum adsorption wavelength of carrot carotenes. Figure 3 shows the calibration curve with a correlation coefficient close to 1.
According this figure, the maximum adsorption length wave is 450 nm in comparison to [26]
The cyclic voltammograms of carrot carotenoids at different scanning speeds are presented in Figure 4(a). It is observed that at low scanning speeds, of the potential (5, 10, 20 mVs−1), the curves are irregular. A more representative
![]()
Figure 2. Scavenging for extract of carotenes from carrots.
![]()
Figure 3. Calibration curve for β-carotene.
voltammogram is obtained when the sweep rate is 50 mVs−1. However, a normal cyclic voltammogram is only obtained at the scan rate of 100mVs−1 (Figure 4(b)). This mode of evolution of the voltammogram as a function of the scanning speed has been obtained by various authors on various organic compounds [27] [28] [29] . The voltammogram obtained at the speed of 100 mVs−1 is similar to those of pure β carotenes [8] , however the peaks are less marked here. This difference can be attributed to the presence of lipids in the medium. The presence of a single anodic peak shows that the electrochemical oxidation reaction of β carotene takes place in a single step. In fact, it has been demonstrated [30] that the oxidation potential of β carotene (Car) and the radical cation (Car●+) are so close that the two reactions take place simultaneously.
et
The low values of
and
show that β-carotene has a high antioxidant
![]()
Figure 4. (a) Cyclic voltammogram of carotene (10−6 M) at the speeds of 5, 10, 20 et 50 mV/S. (b) Cyclic voltammogram of carotene (10−6 M) at the speed of 100 mV/S.
power [28] , the oxidation is determined as quasi-reversible according to the Nicholson-Shain criterion [31] [7] [8] . This antioxidant power is attributed to the presence of conjugated double bonds (Figure 1 performed with AUTOLAB PGSTAT30 potentiostat with GPES software) in the structure of β-carotene.
3.2. Carotene Adsorption Kinetics from Carrots on Clays
Carotene adsorption kinetics were performed to determine the contact time required to reach equilibrium for different adsorbents at different initial concentrations and different temperatures.
3.2.1. Effect of Contact Time on Adsorption Kinetics
The influence of the initial concentration of carotenes in the extract in solution on the kinetics of adsorption on raw, activated and bridged kaolinites was investigated at initial concentrations of 20 mg/L, 40 mg/L and 50 mg/L; the results obtained are shown in Figure 5 (making by using sigma plot logician)
Figure 5 shows that the quantities of carotenes adsorbed per gram of adsorbent gradually increase with contact time and stabilize after approximately 30 minutes [32] . The same appearance has been observed by [33] . The type of treatment applied does not seem to have any influence on the adsorption kinetics of carotenes; on the other hand, there is an influence of the initial concentration on the quantities adsorbed at equilibrium. Indeed, the concentrations adsorbed at equilibrium increase with the initial concentrations regardless of the type of treatment applied as shown in Table 2. This increase in the quantity of
![]()
Figure 5. Adsorption kinetics of carotene from carrots on kaolinites: (a) crude, (b) acid activated (c) bridged; at initial concentrations of 20 mg/L (●), 40 mg/L (○) and 50mg/L (▼) 25˚C.
![]()
Table 2. Effect of the initial concentration on the quantity of carotenes adsorbed at equilibrium.
carotenes adsorbed is however not proportional to the initial concentration, it is very strong between 20 mg/L and 40 mg/L and less marked between 40 mg/L and 50 mg/L; there is probably a trend towards saturation of the adsorption sites.
Acid activated clay performs best; however, it should be noted that the differences in the quantities adsorbed by all the adsorbents used here are small in all cases. Our sample of clay types 1:1 (kaolinites) absorbs for the initial concentrations of 20 mg/L for the raw, bridged and activated acid states respectively 0.875; 0.912 and 0.951 mmol/g for example indicates that it is not a structural phenomenon, but rather a surface interaction [34] . Acid activation causes a slight increase in specific surface area; the bridging leads to a deposit of polycations on the surface of the kaolinites and increase a little bit the basal spacing [35] [36] . The samples are of different texture (specific surface and porosity) and the adsorbed quantities are not always closely related to the texture; it can therefore be concluded that the texture has no significant influence on the adsorption of carotenes.
3.2.2. Influence of Temperature on Adsorption Kinetics
The adsorption kinetics of carotenes at temperatures of 25˚C, 45˚C and 65˚C were carried out. Figure 6 make by using sigma plot logician show the evolution of the quantities of carotenes adsorbed as a function of time for an initial concentration of 40 mg/L.
By observing these curves, we notice that the adsorption is very strong in the first 10 minutes, followed by a gradual evolution until equilibrium. Overall, it can be seen that the adsorption of carotenes is practically not influenced by temperature (Table 3).
![]()
Figure 6. Influence of temperature on the adsorption kinetics of carotenes from carrots on the kaolinites: (a) raw, (b) activated and (c) bridged at initials temperatures of 25˚C (●), 45˚C (○) et 65˚C (▼); initial concentration: 40 mg/L.
![]()
Table 3. Effect of temperature on the quantity of carotenes adsorbed at equilibrium.
3.2.3. Modeling of Carotene Adsorption Kinetics
The modeling of kinetic data can reveal the different molecular steps involved in the process and thus elucidate the adsorption mechanism involved. Among the different models developed to describe the kinetics of adsorption in solution, three are used in most work. These are the pseudo first order models, the pseudo second order model, and the intraparticle diffusion model [37] - [42] . These three models were therefore used here to model the kinetics of carotene adsorption by raw or modified kaolinites.
The velocity equations in their linear forms for the different models are as follows:
- Pseudo first order kinetics:
(2)
- Pseudo-second order kinetics:
(3)
- The intra-particle diffusion model:
(4)
where: qe and qt are the quantities of adsorbed carotenes (mg/g) at equilibrium and at time t respectively.
k1, k2 and kint are respectively the pseudo first order (min−1), pseudo second order (g/µg∙min) and intraparticle diffusion (µmol/(mg∙min1/2)) rate constants respectively.
α is a constant.
The values of k1, k2, kint, qe and the correlation coefficients R2 are reported in Table 4.
The experimental data conform to the pseudo first order kinetic model if the linear regression ln(qe −qt) in function of t has a coefficient of determination R2 > 0.900.
In the case of the pseudo second order model, the linear regression t/qt in function of t must have an R2 > 0.900.
The data conforms to the intraparticle diffusion model if the R2 of the qt versus
![]()
Table 4. Kinetic parameters of raw and modified kaolinites with carrot extracts: initial concentration = 40 mg/L, clay mass = 2 g, extracted volume = 5 ml, T˚ = 25˚C.
regression is greater than 0.900.
In any case, we must always have an “Absolute Average Deviation” (AAD) close to 1 and in addition the value of the Chi-square (χ2) must be low.
The coefficient χ2 has the expression:
(5)
where: qexp (µmol/g) is the quantity of adsorbed carotenes, obtained experimentally;
qcal (µmol/g) is the theoretical quantity of carotenes adsorbed.
Of the three kinetic models that have been tested with the experimental adsorption data, it can be said through the R2 ≥ 0.9 that the adsorption responds equally well to the pseudo first order, pseudo second order models (similar to [43] ) and to the intra-particle diffusion (Table 4).
However, by comparing the calculated qe values of the different models to the experimental qe, the pseudo second order model responds better than the pseudo first order and intraparticle diffusion models to the adsorption of β-carotenes.
The fact that the experimental data conforms to the pseudo-second-order model implies that the adsorption takes place in two steps. Our experimental data are also consistent with the intraparticle diffusion model, it is deduced that the two stages of carotenoid adsorption are respectively the diffusion of carotenoids from the solution to the clay surface and the interaction at the surface.
For the intraparticle diffusion model, the curves generally have three characteristic zones which represent the initial curve zone, the linear zone and the plateau zone. First, the rising curve is the diffusion of the adsorbate through the solution onto the outer surface of the adsorbent or the molecule-solute bond diffusion layer. The second zone represents gradual adsorption, a stage where intra-particle diffusion is at the limit speed (adsorption-desorption dynamism). The third zone represents the final equilibrium, the stage where intra-particle diffusion begins to drop, due to the extremely low concentration of solute remaining in the solution; this third stage exists when the initial concentration of β carotene is high [40] . The intra-particle diffusion model was used to determine the limiting velocity during the adsorption process. If intra-particle diffusion occurs, qt as a function of will be linear; and if the points pass through the origin, then the rate limiting process is only due to intra-particle diffusion. In this study, the intra-particle diffusion line does not pass through the origin, suggesting that intra-particle diffusion is applicable to this system but was not the only rate-limiting step for step of adsorption.
3.3. Adsorption Isotherm
The kinetic study showed that the adsorption equilibrium is reached after 30 minutes. A contact time of 45 minutes was chosen to construct the adsorption isotherms.
3.3.1. Carotene Adsorption Isotherms
Figure 7 (make by using sigma plot logician) show the adsorption isotherms of
![]()
Figure 7. Carotene adsorption isotherms from carrots for (a) raw, (b) activated and (c) bridged kaolinites at temperatures of 25˚C (●) and 45˚C (○), initial concentrations of 40 mg/L.
carrot carotenoids on raw and modified kaolinites.
It is observed that the raw clay which adsorbs less carotenes and even the adsorption is more effective at 45˚C than at 25˚C.
3.3.2. Modeling of Adsorption Isotherms
Langmuir, Freundlich, Temkin and Dubinin-Raduchkevich models are regularly used to model adsorption isotherms in solution [44] [45] [46] . We applied these models to our experimental results.
The linear form of the Langmuir equation has the expression:
(6)
where:
C is the adsorbate concentration at equilibrium (µg/ml);
qm is the quantity of adsorbate adsorbed at equilibrium (µg/mg);
b is the amount of adsorbate adsorbed to the monolayer (mg/g);
KL is Langmuir’s equilibrium adsorption constant (ml/µg).
Carotene adsorption will follow Langmuir’s equation if the coefficient of determination of the linear regression
in function of C is greater than 0.900.
Another essential characteristic of the Langmuir isotherm is the non-dimensional constant called the separation factor or equilibrium parameter RL, defined by [47] by the equation:
(7)
where:
KL is Langmuir’s constant and C0 the initial carotenoid concentration (mg/L). The values of RL indicate the types of isotherms to be as well favorable (0 < RL < 1), unfavorable (RL > 1) as linear (RL = 1) or irreversible (RL = 0).
The linear form of the Freundlich equation is:
(8)
The experimental data will conform to the Freundlich model if the linear regression lnqe as a function of lnCe has an R2 ≥ 0.900.
where:
- KF is a constant relating to the adsorption capacity of the adsorbate.
- n is the Freundlich constant which indicates the degree of affinity between the adsorbent and the adsorbate. The affinity is very strong when n is greater than 10, it is medium for n between 0 and 10 and low for n < 1.
The linear form of the Temkin isotherm has the expression:
(9)
Similarly, the experimental data will conform to the Temkin model if the linear regression q as a function of lnC has a coefficient of determination R2 greater than 0.900.
Where:
(J/mol), is the Temkin constant related to the adsorption energy;
A is the equilibrium constant corresponding to maximum equilibrium (ml/mg);
R is the universal gas constant (8.314 J/mol K);
T is the absolute temperature of the solution (K).
The linear form of the Dubinin–Radushkevich equation is:
.
q is the quantity adsorbed per gram of adsorbent (mol/g);
qDR is the monolayer adsorption capacity (mol/g);
β is the sorption energy constant relative to the average sorption energy per mol of adsorbate that is transferred to the adsorbent surface.
ε is the Polanyi potential and has the expression
(10)
E: Sorption energy (kJ/mol) If 1 < E < 8, then the adsorption is physical; and if 8 < E < 16, we have chemical adsorption.
Table 5 shows about the values of R2 that the numerical data of the adsorption of carotenoids by various adsorbents conform to the Langmuir model which is based on the homogeneous surface and also to the Freundlich and Dubinin Radushkevich models, which admit a heterogeneous surface. The analysis of the adsorbents showed that these materials are heterogeneous. We can however admit a homogeneous distribution of the adsorption sites, as justification for the conformity of the experimental results with the Langmuir model, similar observation was done by [37] .
The RL values obtained are between 0.174 and 0.004 both at 298K and 318K for the adsorption of carotenes on the different clays. This indicates that the adsorption process is favorable for most cases.
The disparate values of the quantity (qm) of carotenes adsorbed in the monolayer deduced from the Langmuir model show the limits of application of this model to the adsorption of carotenoids. Acid activated clay performs best, followed by bridged clay.
The values of n in the Freundlich equation are all less than 1, indicating poor affinity between carotenoids and clays. This low affinity is best expressed by the interaction energies obtained by the Temkin (B < 1) and dubinin-Radushkevich (1 < E < 8) models. These very low values of B and E suggest that the adsorption of carotenoids by our clays is of a physical type with a very strong contribution from Van der Walls bonds. The involvement of Hydrogen bonds would indeed have induced stronger interaction energies of the order of 20 - 40 KJ∙mol−1.
The mechanism would include the interactions of double bonds of the carbon chain of the carotenes with the acid sites (Lewis and Brönsted) of the adsorbents [48] .
3.4. Adsorption-Desorption Isotherms
Desorption experiments are of great practical importance in the application of face masks because it gives long-term insight into the stability of the adsorbed constituent. Figure 8 (make by using sigma plot logician) show the adsorption-desorption isotherms of carotenoids on raw, activated and bridged kaolinites. These isotherms show hysteresis manifested by a difference between the
![]()
Table 5. Values of Langmuir, Freundlich, Temkin and Dubinin-R constants. for the adsorption of carotenes from carrots on raw clays, activated and bridged with AlCl3.
![]()
Figure 8. Isotherms of adsorption (●) - desorption (○) at 25˚C of carotenes from carrots on raw clays (a), activated with HCl (b) and bridged with AlCl13 (c).
adsorption and desorption isotherms. This lack of similarity between adsorption and desorption isotherms due to hysteresis has been observed on French clays [49] [50] . The desorption isotherms are below the adsorption isotherms, indicating that the adsorption of carotenoids is partial and reversible. This reversibility justifies the low interaction energies found previously.
Desorption is physical, however, by comparing the adsorbed and desorbed quantities, it appears from this table that the desorbed quantity represents on average 50% of the adsorbed quantity
4. Conclusion
Experimental carotenoid adsorption data from carrots on crude and modified kaolinites agree equally well with pseudo-first-order, pseudo-second-order and intra-particle diffusion models according the R2; however, by comparing the calculated qe values of the different models to the experimental qe, the pseudo second order model responds better, Therefore the adsorption takes place in two steps. The quantities adsorbed are proportional to the initial concentrations. The increase in the initial concentration leads to an increase in the quantity of β carotene fixed on the clay substrates under the study conditions. In addition, at high temperature, the adsorption rate is not significantly greater and the number of adsorbed carotenoids is substantially similar. Amongst the four model (Langmiur, Freundlich, Temkin and Dubinin-R.) for carotenoid adsorption equilibrium data from carrots in cyclohexane studied, the Freundlich model describe better adsorption with maximum adsorption at the monolayer. Sorption experiments confirm that adsorption is physical.