Etiological and Radiological Profile of Acute Lower Respiratory Infections during the Pre-COVID Period in the Paediatric Ward of the Teaching Hospital of Mali and in the Community Health Centre of Yirimadio in Bamako ()
1. Introduction
Predisposing drivers in children include immature immune system as well as others, such as malnutrition and overcrowding. In adults, smoking is the most important risk driver [5]. Bacteria are a major cause of lower respiratory tract infections, with Streptococcus pneumoniae being the most common cause of community-acquired bacterial pneumonia in many countries. However, most acute respiratory infections are caused by viruses or a mixture of viral and bacterial infections [2] [6] [7] [8]. Many parameters are taken into account in pneumonia diagnosis: a complete blood count, a sputum culture and a chest X-ray [9]. The chest X-ray shows normal signs or areas of bilateral, unilateral or uneven consolidations, nodular opacities and bronchial wall thickening. It can be difficult to establish difference between pneumonia since infection cause cannot be reliably determined from imaging appearance [9].
This test targets the 13 serotypes included in the 13-valent conjugate vaccine and 8 additional key serotypes.
The new virological diagnosis tools enable to refine epidemiological data. Compared with immunohistochemistry and cultures, multiplex Polymerase Chain Reaction (PCR) techniques reveal much broader viral spectrum, including already known organisms, but also so-called “emerging” viruses most often arising from genetic recombination [7] [10]. In addition, real-time PCR test consisting of 7 triplexed reactions helps to identify 11 individual serotypes and 10 smaller serogroups representing the majority of Streptococcus pneumonia pathogenic isolates. This test targets the 13 serotypes included in the 13-valent conjugate vaccine and 8 additional key serotypes [11].
Very little studies have been completed in Mali to reveal bacteriological and or virological evidence of this disease. The current study aims examining standard x-ray etiological aspects of acute lower respiratory infections in the pediatric department of the Hôpital du Mali and the Community-based Health Center of Yirimadio.
2. Equipment and Methods
2.1. Study Type and Period
It is about a descriptive prospective study carried out from January to December 2018 (12 months).
2.2. Study Venue
This descriptive study was conducted at the pediatric department of Hôpital du Mali and at the Community-based Health Center of Yirimadio.
2.3. Sampling
This study focused on patients in all age-groups of both sexes referred to our service by Hôpital du Mali pediatric department (from 6 months to 15-years old) and the Yirimadio Community-based Health Center (children and adults) for lower respiratory infection.
2.3.1. Inclusion Criteria
Any patient of any age and of both sexes referred to the x-ray department by the pediatric department of Hôpital du Mali and the Yirimadio Community-based Health Center for a lower respiratory infection during the study timeframe.
2.3.2. Non-Inclusion Criteria
We excluded from this study:
· patients suffering from chronic respiratory infections:
· patients from structures other than the pediatric service of Hôpital du Mali and the Community-base Health Center of Yirimadio; and
· patients for whom we have not obtained assent or consent for their participation.
2.4. Variable
Variables studied were of four kinds:
· socio-demographic data (age, gender);
· clinical data (cough, temperature, dyspnea, breath sounds);
· chest x-ray results (syndromes: bronchial, alveolar, interstitial, pleural, mediastinal); and
· biology results (hemogram, PCR).
2.5. Study Methods
2.5.1. Clinical Methods
Children were examined through axillary temperature measuring, physical and functional respiratory signs recording involving: respiratory rate, moist breathing, struggle signs presence, nasal discharge. A procedure has been drafted relating to clinical examination.
2.5.2. Biological Methods
1) Complete blood counts
The counting of formed blood elements is done by hematological processors after whole blood sampling on a tube containing an anticoagulant, preferably of EDTA type. Leukocyte formula was established manually by reading blood smears stained with May Gruwal Giemsa MGG.
2) Collection and treatment of airway secretions
Samples were collected by nasal or pharyngeal swab and transported to Charles Mérieux Infectiology Center for analysis.
Nucleic acids were extracted using viral RNA extraction kit (QIAGEN, Duesseldorf, Germany) with 200 μl of VTM. Real-time multiplex PCR for the detection of 19 viruses and five bacteria (Fast-track Diagnostic Respiratory Pathogens 21 PLUS, Fast-track diagnostic, Esch-sur-Alzette, Luxembourg) was used to screen for respiratory pathogens, including the virus influenza A (IFVA), IFVA/H1N1 (swine line), influenza B virus (IFVB); human coronaviruses (HCoV) NL63, 229E, OC43 and HKU1, human parainfluenza virus (HPIV) 1, 2, 3 and 4, human metapneumovirus A and B (HMPV), human rhinovirus, RSV A and B, human adenovirus (HAdV), enterovirus (Ev), human parechovirus, human bocavirus (HBoV), Mycoplasma pneumoniae (M. pneumoniae), Chlamydophila pneumoniae (C. pneumoniae), S. pneumoniae, Hib and Staphylococcus aureus (S. aureus). Samples positive for S. pneumoniae were typed using previously published triplex real-time PCR method including 21 common pneumococcal serotypes.
2.5.3. X-Ray Methods
1) Equipment
· X-ray machine: WDM brand, year 2009;
· Operating console: behind which medical staff is isolated from the rest of the room by a lead-plated protective-storm glass;
· A printer: AGFA DRY STAR 5503.
· Digitizer: image semi-digital development.
2) Examination performing technique: examination was achieved without preparation.
· With adults: examination was made in a standing position, shirtless, deep inspiration, the front part of the trunk attached to the capture plate and the x-ray in his back; this is called a postero-anterior grip.
· With Newborns and small children: examination was made in the supine position, the plate is placed in the back; we call it antero-posterior grip
· Success criteria:
○ Centering: visualize the entire rib cage;
○ Symmetry: visualization of clavicles internal edges at equal distance from the dorsal spines;
○ Release: visualization of scapulae outside lung parenchyma;
○ Deep breathing in: 7 anterior costal arches and 10 posterior costal arches above the diaphragmatic domes;
○ Visualization of stomach air pocket below left dome.
3) Reading: X-rays were interpreted by senior and assigned radiologists.
2.6. Data Analysis
Data collection was done using an individual survey sheet on which socio-epidemiological, clinical and x-ray data was recorded. Data was entered and analyzed using SPSS version 22.0 software. Word processing was done with Microsoft Word 2013 software. Proportion comparisons were made by Chi-square test and significance level was set at p < 0.005.
2.7. Ethical Aspects
The hospital officials’ authorization and that of children’s parents were to be obtained to use the data. Patient’s anonymity and confidentiality were respected in accordance with medical ethics rules and the legislation on biomedical and scientific research. There is no conflict of interest in this study. Data integrity was respected.
3. Results
From January to December 2018, 37,950 patients consulted in the two facilities, among whom we diagnosed 453 cases of acute lower respiratory infection meeting our inclusion criteria, i.e. a frequency of 1.19%.
3.1. Socio-Demographic Characteristics
The 0 - 5 age group was the most represented (64.5%). Females were slightly predominant (50.6%) with a sex ratio of 0.97 (Table 1).
3.2. Clinical Features
Cough was the most frequent clinical sign in our series (98.7%) followed by fever (58.9%) and crackles (28.5%) (Figure 1).
![]()
Table 1. Distribution of patients according to age group and Gender.
![]()
Figure 1. Distribution of patients according to clinical signs.
3.3. Radiographic Characteristics
The standard chest X-ray was pathological in 70% of our patients. Bronchitis was present in 75.4% of cases (Table 2).
3.4. Biological Characteristics
The majority of our patients (53.9%) had an abnormal white blood cell count. These were hyperleukocytosis in 35.5% and leukopenia in 18.4%. PCR was positive in 83.9% of our patients. It revealed a co-infection in 52.5% of patients. Virus/bacteria co-infection was the most frequent (46.8%) (Table 3).
Streptococcus pneumonia was the most represented bacterium with a frequency of 87.6% followed by Staphylococcus aureus (24.9%) (Figure 2).
Human rhinovirus (HRV) was the most represented virus with 17%, followed by influenza A and B virus (FLU A&B) (7%) and human para influenza virus (7%) (Figure 3).
The combination of Streptococcus pneumoniaa and human rhinovirus was the most represented type of co-infection with 7.5% (Figure 4).
![]()
Table 2. Distribution of patients according to radiographic findings.
![]()
Table 3. Distribution of patients according to biological results.
![]()
Figure 2. Distribution of patients by type of bacteria.
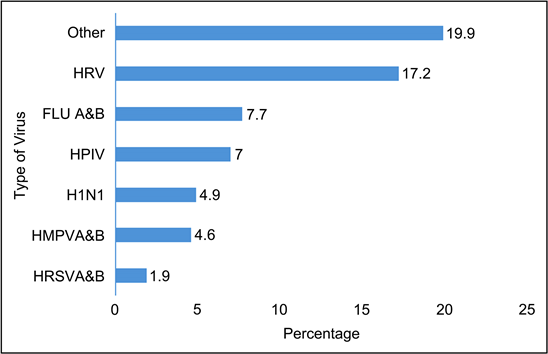
Other: COV 229 (0.2%), FLU A&B (0.2%), FLU A&B + EV (0.2%), H1N1 + HRV (0.2%), H1N1 + FLU A&B (0.2%), HAdV + EV (0.2%), HAdV + H1N1 (0.2%), HAdV + HBoV (0.2%), HAdV + HBoV + HRV (0.2%), HAdV + NL63 + HRV (0.2%), HBoV + EV (0.2%), HboV + FLU A&B (0.2%), HCOV 229E + HPIV (0.2%), HCOV 229E + HRV (0.2%), HCOV NL63 + HAdV(0.2%), HCOV NL63 + HMPVA&B (0.2%), HCOV NL63 + HPIV (0.2%), HCOV229E + HAdV + EV (0.2%), HCOVOC43 + HRV (0.2%), HMPVA&B + FLU A&B, + H1N1 + EV(0.2%), HMPVA&B + HCOV HKU1 (0.2%), HMPVA&B + HPeV (0.2%), HMPVA&B + HRSVA&B + HPIV (0.2%), HPIV + HAdV + HBoV (0.2%), HPIV + HAdV + HRV (0.2%), HPIV + HBoV (0.2%), HPIV + HBoV + EV (0.2%), HRSVA + EV (0.2%), HRSVA + HAdV (0.4%), HBoV + H1N1 (0.4%), HCOV NL63 (0.4%), HMPVA&B + EV (0.4%), HMPVA&B + HBoV (0.4%), HMPVA&B + HBoV + HRV (0.4%), HMPVA&B + HPIV (0.4%), HPIV + HAdV (0.4%), HBoV + FLU A&B (0.7%), HCOV HKU1 (0.7%), HMPVA&B + HAdV (0.7%), HPIV + EV (0.9%), HAdV + HRV (1.1%), HBoV (1.1%), HBoV + HRV (1.3%), EV (1.5%), HAdV (1.5%), HRSVA (1.9%).
Figure 3. Distribution of patients by virus type.
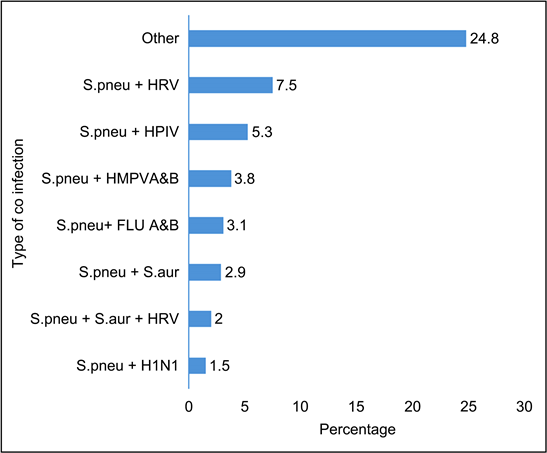
Other: FLUB + EV (0.2%), FLU A&B + HBoV (0.2%), H1N1 + HRV (0.2%), HBoV + HRV (0.2%), HCOV229E + HAdV + EV (0.2%), HMPVA&B + HBoV (0.2%), HMPVA&B + HBoV + HRV (0.2%), HMPVA&B + HCOV HKU1 (0.2%), HPIV4 + HRV (0.2%), HRSVA + HAdV, S.aur + HAdV (0.2%), S.aur + HCOV 229E + HPIV (0.2%), S.aur + HCOV NL63 + HPIV, S.aur + HMPVA&B (0.2%), S.aur + HMPVA&B + HAdV (0.2%), S.aur + HMPVA&B + HRSVA&B + HPIV (0.2%), S.aur + HMPVAB + HPIV (0.2%), S.aur + HPIV (0.2%), S.aur + HPIV + HBoV (0.2%), S.pneu + C.pneu (0.2%), S.pneu + COV 229 (0.2%), S.pneu + FLU A&B + H1N1(0.2%), S.pneu + FLU A&B + HIB (0.2%), S.pneu + HAdV + EV (0.2%), S.pneu + HAdV + H1N1 (0.2%), S.pneu + HAdV + HBoV (0.2%), S.pneu + HAdV + HBoV + HRV (0.2%), S.pneu + HAdV + NL63 + HRV (0.2%), S.pneu + HCOV 229E + HRV, S.pneu + HCOV HKU1 (0.2%), S.pneu + HCOV NL63 + HAdV (0.2%), S.pneu + HCOVOC43 + HRV, S.pneu + HIB + H1N1 (0.2%), S.pneu + HIB + HRV, S.pneu + HMPVA&B + EV, S.pneu + HMPVAB (0.2%), S.pneu + HMPVAB + EV, S.pneu + HMPVAB + H1N1 + EV + FLU A&B (0.2%), S.pneu + HMPVAB + HAdV, S.pneu + HMPVAB + HBoV (0.2%), S.pneu + HMPVAB + HPeV, S.pneu + HPIV + HAdV, S.pneu + HPIV + HAdV + HBoV, S.pneu + HPIV + HBoV + EV, S.pneu + HPIV + HRV + HIB (0.2%), S.pneu + HPIV + HAdV (0.2%), S.pneu + HRSVA + EV (0.2%) (0.2%), S.pneu + S.aur + FLU A&B + HBoV (0.2%), S.pneu + S.aur + H1N1 (0.2%), S.pneu + S.aur + HAdV (0.2%), S.pneu + S.aur + HAdV + HRV (0.2%), S.pneu + S.aur + HBoV + EV (0.2%), S.pneu + S.aur + HMPVA&B + HBoV + HRV (0.2%), S.pneu + S.aur + HPIV + HAdV + HRV (0.2%), S.pneu + S.aur + HPIV + HRV (0.2%), S.pneu + S.aur + HPIV3 + HRV (0.2%), S.pneu + S.aur + HPIV4 + EV, S.pneu + S.aur + HRSVA&B (0.2%) (0.4%), S.aur + H1N1, S.pneu + C.pneu + HPIV, S.pneu + FLUB + HBoV (0.4%), S.pneu + HBoV, S.pneu + HBoV + H1N1 (0.4%), S.pneu + HIB + HMPVA&B (0.4%), S.pneu + S.aur + HPIV (0.4%), FLUB + HIB (0.7%), S.aur + FLU A&B (0.7%), S.pneu + EV (0.7%), S.pneu + HAdV (0.7%), S.pneu + HPIV + EV (0.7%), S.pneu + HAdV + HRV (0.9%), S.pneu + HIB (0.9%), S.pneu + HPIV + HRV (0.9%), S.pneu + S.aur + FLU A&B (0.9%), S.aur + HRV (1.1%), S.pneu + HBoV + HRV (1.1%), S.pneu + HRSVA (1.1%).
Figure 4. Distribution of patients by type of co-infection.
4. Discussions
4.1. Overall Frequencies
From January to December 2018, 37,950 patients consulted in the 2 facilities among whom we diagnosed 453 cases of acute lower respiratory infection meeting our inclusion criteria i.e. a frequency of 1.19%. Our frequency was similar to that of Chen et al. [12] who reported a frequency of 1.4% in children in displacement camps. It was lower than that of Kabamba et al. [13], Yoseph Alameh [14] and Nzamé et al. [15] who respectively recorded frequencies of 26.1%, 20.6% and 10.9% among children.
4.2. Socio-Demographic Characteristics
· Age
The majority of patients in our series were children, i.e. a proportion of 80.6%. Children under 5 years of age were the most represented with a frequency of 64.5%, followed by children [5 - 9 years] (10.4%) and children [10 - 15 years] (5.7%) for an average of 9.627 ± 15.228 years and extremes of 1 month to 78 years.
Our result was comparable to the data in the literature (pneumo) [16] [17] [18] [19] and to that of Chabane M [4] in 2016 who reported a high representation of the youngest (Newborn and Infant) 67%, followed by small children (16%) and older children (13.8%).
This is probably due to organic factors, the immaturity of their immune system and nutritional status, or to the fact that young people and adults opt for self-medication as a first choice for this type of infection.
· Gender
There was no significant difference between the two sexes in our study with a sex ratio of 0.97 in favour of women. However, most African authors have observed a predominance of males in their studies, notably Nzamé [15], Kabamba [13] and Nagoan et al. [20], who reported sex ratios of 2 and 1.2 respectively in favour of boys. These data would indicate that gender is not an important risk factor in lower respiratory tract infections.
4.3. Clinical Characteristics
In our series, cough was observed in almost all our patients (98.7%). It was associated with fever in 58.9% of our patients, with sibilant rales in 28.5%, with crepitus in 25.6% and with dyspnoea in 15%. Our results were similar to the data in the literature [21] [22] [23] [24] and those reported by most authors. In the study by Chabane M [4], the main signs were fever, respiratory discomfort and cough in 65.5%, 15.9% and 13.8% of cases respectively. However, Nzamé et al. [15] observed fever in 94.4% and cough in 87.5%.
4.4. Radiological Characteristics
The standard chest X-ray performed in our patients was pathological in 70% of them, far superior to the results of Bach et al. [25], and Mwananyanda et al. [26] who respectively obtained 42% and 40.5% of pathological chest X-ray in patients with lower respiratory infections in their studies.
These were bronchitis in 75.4% of cases, pneumonia in 13.5% of cases and bronchopneumonia in 12.3% of cases. Our data are close to those of Guittet et al. [27] who found a high frequency of bronchitis in 38.4% of the patients in their study followed by pneumonia (11.9%). This is different from Bach et al. [25] who reported bronchoalveolar syndrome in 46% of patients and pneumonia in 4%. They are also different from those of Nagoan et al. [20] who found, in order of frequency, alveolar syndrome (70.3%) and bronchoalveolar syndrome (29.7%).
4.5. Biological Characteristics
The majority of our patients (53.9%) had an abnormal white blood cell count. These were hyperleukocytosis in 35.5% and leukopenia in 18.4%. This result was close to that of Odièvre [28] who found hyperleukocytosis in one of three subgroups in a paediatric study consisting of three subgroups.
PCR came back positive in 83.9% of our patients. Our result was close to that of Koenig et al. [7] who observed a positivity rate of 83% in their patients. It was slightly lower than that of Berrajah et al. [29] who had a positive PCR in 86.7% of the patients in their series. However, it was higher than that of Appak et al. [30] who found a frequency of 58.7% in their study.
PCR revealed co-infection in the majority of our patients (52.5%), bacterial infection in 16.1% and viral infection in 15.2%. This frequency was higher than that of Berrajah et al. [29] and Appak et al. [30], who found frequencies of 40% and 10.2% respectively in their series.
Virus/bacteria co-infection was the most frequent (46.8%) while Appak et al. [30]. observed a predominance of the viral combination in their study. It was mostly a combination of Streptococcus pneumoniaa and human rhinovirus (7.5%) in contrast to that reported by Appak et al. [30]. who frequently detected the rhinovirus/enterovirus combination in their series.
The pathogens isolated in order of frequency were Streptococcus pneumoniaa (87.6%) followed by Staphylococcus aureus (24.9%) and human rhinovirus (17%). Our data differ from those of Heather Zar et al. [31] who reported a predominance of Bordetella pertussis (OR 11-08, 95% CI 1-33-92-54), followed by respiratory syncytial virus (8-05, 4-21-15-38) and influenza virus (4-13, 2-06-8-26) in their study of pneumonia in children in South Africa. This differed from Mwananyanda et al. [26] who found in their Zambian series a high frequency of respiratory syncytial virus [26.1%, 95% credibility interval (CIr) 17.0 - 37.7], Mycobacterium tuberculosis (12.8%, 95% CIr 4.3 - 25.3) and human metapneumovirus (12.8%, CIr 6.1 - 21.8) among children with pneumonia.
In our sample the viral causes were dominated by human rhinovirus (17%), followed by influenza A and B virus (7%) and human para influenza virus (7%). This result is close to that of Appak et al. [30], who isolated rhinovirus/enterovirus (36.2%) followed by respiratory syncytial virus (19%) and influenza A/B virus (14.7%).
This is different from Berrajah et al. [29] who found a high frequency of respiratory syncytial virus (42.7%) followed by rhinovirus (32.9%) and adenovirus (28.5%).
4.6. Limitations and Difficulties
Our study was faced with certain limitations and difficulties, which were mainly the loss of patients due to breakdowns of the X-ray machines and strikes of the hospital doctors.
5. Conclusions
It appears that lower respiratory infections are frequent at the Yirimadio Community-based Health Center and at the UHC Hôpital du Mali. Children were the most affected social layers. Cough was the most common reason for consultation. It also emerged that bronchial syndromes were the most frequent radiological lesions and that the most represented infectious agents were virus-bacteria association. It was most often a combination of Streptococcus pneumonea and human rhinovirus.
Although the x-ray results alone are not sufficient for pneumonia definite diagnosis, they can in association with the clinical and biological results improve diagnosis accuracy of this disease.