1. Introduction
In the long process of human understanding of the world, the issue of matter and energy has attracted many scholars and puzzled many wise men.
In the last century, there were two outstanding scientists in physics.
One is Planck, a German physicist and founder of quantum theory, who proposed the energy quantum formula. It is now called Planck’s law.
In order to study the law of energy absorption and release of blackbody, physicists Rayleigh, Jens and Wayne proposed two formulas respectively.
But compared with reality, the Rayleigh-Kings formula is only suitable for the infrared range. Wayne’s formula is only suitable for the ultraviolet range.
Planck has worked hard for several years to derive an energy formula suitable for the range from infrared to ultraviolet.
Planck put forward the concept of basic quantum, which is now called Planck constant.
Planck believes that if the energy absorbed and released by the black body is discontinuous, one by one, then the calculated energy is very accurate and practical.
Such a share of energy, that is, energy quantum, is discontinuous.
In 1900, the Bulletin of the German Physical Society published Planck’s argument entitled “On the Improvement of Wien’s Spectral Equation”.
Later, Planck proposed a new energy formula [1] - [6]
(1.1)
(1.1) is called the energy quantum formula, and is now called Planck’s law.
Among them,
E is the quantum of energy. J·s, Einstein believed that hv is the energy of a photon
Planck is called the fundamental energy quantum, and is now called the Planck constant.
v is the frequency.
For example
v = 1018,
Calculated by (1.1)
Planck’s law not only plays an important role in quantum mechanics, but also effectively promotes the research of modern physics, atomic physics, nuclear physics, chemistry, and other disciplines.
However, the principle of Planck constant h needs to be further studied.
If it is allowed to change the understanding of hv, that is, the energy quantum is known as v photons, then the Planck constant should be the kinetic energy of a photon.
Another scientist is Einstein, an American German, a great physicist, and the founder of special relativity and general relativity.
Einstein believed that matter and energy can be converted. It has changed the traditional concept of matter and energy. It is the change of human thinking methods.
The photoelectric effect is an epoch-making discovery in physics. However, physicists have encountered difficulties in theoretical research of photoelectric effect.
Einstein put forward the quantum of light, that is, photon, and got the equation of photoelectric effect:
where hv represents the energy of a photon. W is the energy of electron detachment.
The maximum initial kinetic energy after electron separation
It successfully interprets the photoelectric effect.
It has been proved that light is a particle.
Then, the energy discontinuity of the blackbody is applied to space.
Thus, physicists have a new understanding of the particle and wave phenomena of light, which is the wave-particle duality of light.
Einstein believed that the study of the quantum of light is hopeful to explain various difficult problems of quantum mechanics.
Recently seen:
International Journal of Astronomy and Astrophysics > Vol.12 No.3, September 2022.
https://www.scirp.org/journal/ijaa/
Scalar Field Model Provides a Possible Bridge between General Relativity and Quantum Mechanics Rickey W. Austin Researcher at Saint Claire Scientific Research, Albuquerque, USA.
The relationship between relativity and quantum mechanics is described.
We find that the photoelectric effect equation is also related to quantum mechanics.
Since photon energy can be converted into electron kinetic energy, on the contrary, electron kinetic energy can also be converted into photon energy. Then the electron should also have the characteristics of photon, and also have the wavelength. According to the photoelectric effect equation, we can get
The wavelength of the electron is thus obtained
Therefore, from the photoelectric effect equation, we can recognize the wave-particle binary characteristics of physical particles. According to another photoelectric effect equation:
Get
Can get
From this, we can get
According to this formula, it can be confirmed that no matter how high the frequency of light is, the speed of electrons is less than the speed of light.
Also
Journal of Modern Physics > Vol.14 No.3, February 2023
https://www.scirp.org/journal/jmp/
Hubble Tension versus the Cosmic Evolution of Hubble Parameter in the Unicentric Model of the Observable Universe
Ahmad Hujeirat IWR, Heidelberg University, Heidelberg, Germany.
Put forward new understanding:
Curved space-time can evolve into flat space-time. This shows that space-time is both curved and flat. This is the dialectical understanding of general relativity.
And inspire another new understanding:
Usually, the speed of material movement is relative. The speed of light wave is absolute.
Therefore, the speed of material movement is both relative and absolute. This is the dialectical understanding of special relativity.
In 1905, the German Yearbook of Physics published Einstein’s argument on the electrodynamics of moving bodies.
In the special theory of relativity, Einstein proposed the conversion formula of mass and energy [6] - [11]
(1.2)
(1.2) is called Einstein’s mass and energy equation.
This equation shows that matter can be converted into energy.
Energy can be converted into matter. Among them,
E is energy. J∙s.
m0 is the static mass. kg.
light speed c = 299,792,458 ± 0.1 M/s,
The physical meaning of the mass-energy equation is:
The energy “converted” by the mass m0.
For example
Let m0 = 2 kg, c = 299,792,458 ± 0.1 M/s,
Calculated by (1.2)
The mass-energy equation is related to Planck’s law.
If it is allowed to change the understanding of hv. That is, if the energy quantum is known as v photons, then the principle of Planck constant can be obtained from the mass-energy equation. In this way, the mass-energy equation may become the basis of quantum mechanics.
Einstein predicted that mass could be converted into huge energy.
The success of the nuclear bomb not only confirmed Einstein’s prediction, but also proved that the mass-energy equation was correct.
Now physicists have realized that:
Hydrogen condenses into helium, releases huge energy and releases photons, which are stars.
What is the opposite? That is:
Helium splits into hydrogen, absorbs huge energy and absorbs photons. What is that? At present, it is not clear.
It may be a certain type of black hole.
Oster found that the wire was electrified and formed motion. But the opposite situation has not been studied.
Since the wire is electrified and forms movement, what does the wire movement form?
Faraday found that the wire moves and forms electric energy according to the research of Oster. As a result, mankind has entered a brilliant era of electric energy.
Therefore, the study of the opposite situation may evolve into a great change in physics.
According to the mass-energy equation, the changing mass can be converted into energy. Changing energy can be converted into mass.
The constant change of mass and energy, like the constant change of electric field and magnetic field, has the characteristics of wave.
The minimum distance between mass and mass is one wavelength λ.
The number of changes in mass and energy per second is the frequency v.
The distance of matter per second is λv.
Therefore, the change of mass and energy is characterized by waves. This is the wave of physical particles.
2. Debroy Wavelength Formula
In 1924, physicist De Broglie, based on Planck’s law and Einstein’s mass-energy equation, through theoretical study of Bohr’s electron orbit, believed that not only the bose particles have the characteristics of waves, but also the physical particles have the characteristics of waves. And it was recognized by Einstein.
Let’s look at the relationship between bose particles and physical particles.
It can be obtained from Planck’s law (1.1) [12] [13] [14] [15] :
This is the energy of the photon. Compare Einstein’s mass and energy equation,
This is the energy converted by physical particles. According to the conversion of mass and energy, it can be confirmed that:
The energy of bose particle λ is related to that of physical particle m0, thus:
The momentum of physical particle conversion is obtained
We can get the wavelength formula:
This is the principle of Debroy wavelength.
For example
Let m0 = 2 kg, c = 299,792,458 M/s, h = 6.62607015 × 10−34 J∙s.
Calculation
This principle shows that the de Broy wavelength can be obtained by giving the energy formula of the physical particle.
The energy formula of physical particles [16] [17], including Einstein’s mass-energy equation:
Einstein’s kinetic energy formula for the mass of motion:
Einstein’s mass and energy formula of motion mass:
The kinetic energy formula of the equivalent moving mass:
Mass and energy formula of equivalent motion mass:
Based on the Debloy wavelength principle, it can be generalized to the general Debloy wavelength.
In order to adapt to the general situation of matter waves, the static mass can be extended to the moving mass.
Einstein believed that matter moves and mass expands.
Let’s look at Einstein’s motion mass formula [18] [19] [20],
Among them,
mu is the motion quality. m0 is the static mass. Lorentz factor
The speed of matter movement u, the speed of light c, obviously, matter movement, mass expansion.
Based on Einstein’s mass of motion, we can use the kinetic energy formula to obtain the kinetic energy as [21] [22] :
Based on Einstein’s motion mass, we can also get the changing mass Δm, and then we use the mass energy equation to convert it into energy.
Let’s look at the mass of the motion change of the object.
According to the motion quality, we can get the changing mass:
The changing mass is converted into energy, and the mass energy formula is obtained:
Obviously, the energy obtained by the kinetic energy formula is equivalent to the mass and energy formula converted by the changing moving mass.
From the above, we can get
Partial calculation
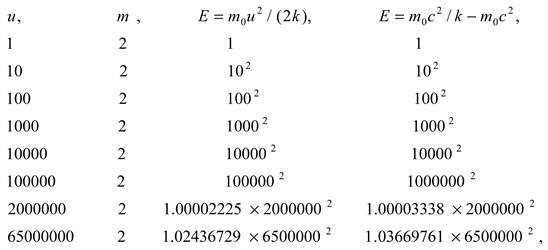
In a large speed range, obviously, the kinetic energy formula
And mass energy formula
is equivalent, and can be converted into the wavelength formula of physical particles.
In addition, according to Einstein’s motion mass formula
The Lorentz factor can be converted into an equivalent motion mass formula [23]
(2.1)
Partial calculation
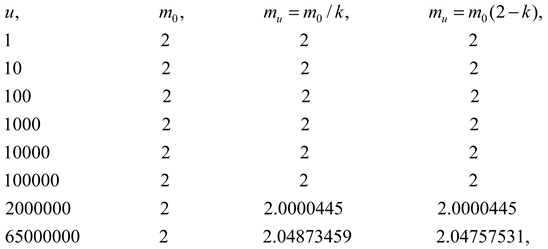
It is equivalent in a large speed range.
We can learn from the previous method, according to the motion quality
Equivalent motion mass
The kinetic energy formula is obtained:
According to (2.1) and kinetic energy formula, another kinetic energy formula can be obtained [22] [23]
We can use the kinetic energy formula and the mass energy formula to discuss the matter wave. From the kinetic energy formula, we can get:
From this, we can get [23] [24]
(2.2)
This is the common Debroy wavelength. Suitable for wavelength calculation from low speed to light speed.
For example
Let m0 = 2 kg, u = 100 M/s,
Calculated by (2.2)
To confirm this wavelength, we can calculate the energy by substituting Planck’s law:
This is the same as the calculation of kinetic energy formula:
Set electronic quality
m0 = 9.1 × 10−31 kg, u = 5.1 × 107,
By (2.2) get
This is very close to the actual test wavelength of the electron beam hitting the crystal:
To confirm this wavelength, we calculate the energy by substituting Planck’s law:
This is the same as the calculation of kinetic energy formula:
It is also suitable for wavelength calculation of light speed.
According to (2.2), u = c, get
We can also get another Debroy wavelength based on the minimum mass.
3. Minimum Mass of Material Wave
According to the general wavelength of Debroy (2.2), the mass formula is obtained [22] [23] [24] [25] :
(3.1)
Among them,
Static mass m0, kg,
Wavelength λ,
Speed u, M/s,
Light speed c = 299,792,458 M/s,
Planck constant
.
Let’s look at the minimum mass.
According to (3.1), it is obvious that the larger the wavelength and speed, the smaller the mass.
For example
Let λ = 10,000 M, u = 100,000,000 M/s, c = 299,792,458 M/s, h = 6.62607015 × 10−34 J∙s.
Calculated by (3.1)
It is far less than the mass of the electron. But it is not the smallest particle.
According to (3.1), we can confirm that,
If the λu There is a maximum value, then the m0 with minimum value [24] [25].
Obviously,
,
, and
,
The minimum value can be obtained
By minimum energy
Get the minimum mass [24] [25] [26] :
(3.2)
Let
, calculation:
(3.2) is defined as the fundamental mass quantum.
The fundamental energy quantum corresponds to the fundamental mass quantum.
4. New Wavelength Formula
Let’s look at the change rule of mass and energy. The change of mass µ corresponds to the change of energy h.
Let’s look at the mass of object motion change Δm,
According to (2.1)
You can get the motion quality:
Among them,
m0 is the static mass. And the quality of change:
From this, we can get
Convert it into energy and get the mass and energy formula
And (2.1)
Acquired
is equivalent.
According to Planck’s law and mass energy formula
The wavelength formula is obtained [24] [25] [26] [27]
(4.1)
It is obtained by substituting (3.2) into (4.1)
That is
(4.2)
Here (4.2) is the new wavelength [26] [27] [28].
The same wavelength formula as (2.2)
For example
Let m0 = 2 kg, u = 100 M/s, c = 299,792,458 M/s,
,
Calculated by (4.2)
Set electronic quality
m0 = 9.1 × 10−31 kg, u = 5.1 × 107,
By (4.2) get
This is the same as the calculation of De Broy’s universal wavelength (2.2).
Based on the new wavelength formula, the frequency formula can be obtained.
5. Generalized Planck’s Law
According to the new wavelength formula, the frequency formula of matter wave can be obtained [28]. By (4.2) Obtained frequency:
(5.1)
The frequency formula in (5.1) can be applied to the calculation of Planck’s law.
(5.1) Substitute Planck’s law (1.1) to obtain another energy formula [28] [29] [30] [31] :
(5.2)
(5.2) is the energy formula of matter wave. Among them,
And
µ is the fundamental mass quantum [32].
m0 is the static mass of the physical particle.
is the mass of physical particle motion.
Bose particles are considered to have no static mass, but have a moving mass, which is also suitable for energy calculation of Bose particles.
In this way, the energy formula of matter wave is suitable for the energy calculation of all substances.
For example
Let m0 = 2 kg, u = 100 M/s, c = 299,792,458 M/s,
,
.
Calculated by (5.1)
This is the same as the calculation of kinetic energy formula:
Let the motion mass of photon
,
,
,
.
Calculated by (5.1)
Calculation by substituting Einstein’s mass and energy equation
Get
In the above discussion, the key is the basic mass µ, which is a constant. What is the relationship between this constant and Planck’s constant?
If this constant is the static mass of the photon, then Planck’s constant is the kinetic energy of the photon:
It is calculated that h = 6.62607014657, which is Planck constant.
Let’s take a look at physicists’ actual test of the static mass of photons.
International physicists have measured that the static mass of photons is less than 10−48 kg.
Academician Luo Jun of the Chinese Academy of Sciences measured that the photon static mass is less than 1.2 × 10−54 kg.
If the physicists measure that the photon static mass is less than 10−48 kg, greater than 10−57 kg.
It can be confirmed that µ is the static mass of photons.
Planck’s law is the energy of v photons:
Einstein’s equation of mass and energy is the basis of quantum mechanics.
The energy calculation of physical particles and bose particles is unified as the generalized Planck’s law.
6. Conclusions
According to Planck’s law
And Einstein’s mass and energy equation
Based on Einstein’s motion mass formula, the general Debroy wavelength formula is obtained
(6.1)
convert to frequency formula according to (6.1) [29] [30] [31] [32].
We get the generalized Planck’s law
(6.2)
among
, and
,
For example
Let
, u = c,
.
Calculated by (6.2)
This is Planck’s constant.
According to (6.2), substances can be expressed in the form of waves [22] [25] [30] [32].
Matter has wave-particle duality.
Acknowledgements
The scientific research team of academician Luo Jun of the Chinese Academy of Sciences tested the mass of physical particles.
Suggestions put forward by Professor Mei Xiaochun, a visiting scholar of Peking University.