How Does Heat-Stress Intensity Affect the Stability of Microbial Activity and Diversity of Soil Microbial Communities in Outfields and Homefields’ Cultivation Practices in the Senegalese Groundnut Basin? ()
1. Introduction
Many ecosystem services such as nutrient cycling, primary production and carbon sequestration are essential soils’ functions [1] . The need to study how soil microbial communities respond to climate-related disturbances (i.e., heat stress and drought) is urgent because soil microorganisms are involved in many biogeochemical cycling processes that are influenced by the main environmental factors, such as temperature and moisture [2] [3] . The taxonomic stability of soil microbial communities, specifically, is important for maintaining soil functions [4] . Thus, the combination of resistance (RS) and resilience (RL) determines the ability of a community to continue to function under changing conditions [5] . RS and RL of soil microbial communities are two main components of ecological stability that are used to evaluate the communities’ responses to disturbances [6] [7] . Ng et al. [8] , define RS as the microorganisms’ ability to maintain activity, and RL as their ability to recover. To assess the resistance and the resilience of soil functions, several indices have been developed [5] .
In Senegal’s Groundnut Basin region, the combination of climate change and anthropogenic pressure has already accelerated ecosystem degradation and induced profound changes in the cultivation system [9] . The climatic changes themselves have disrupted the functioning of agroecosystems [10] [11] , including the biological functioning of soils [11] .
Previous studies reported that heat stress reduces the resistance of microbial biomass [1] . In addition, metabolic rates of soil microorganisms reportedly decrease above 40˚C [12] . Recent studies emphasize that the response of microorganisms to heat stress depends not only upon the duration of the stress [13] [14] , but also upon microbial diversity [4] and the soils’ physico-chemical properties [15] [16] [17] [18] . Moreover, Kaisermann et al. [19] , indicate that the RS and RL of soil microbial communities can be affected by soils’ nutrient availability and content of soil organic matter (SOM). Both of these characteristics are known to vary with agricultural practices. Kuan et al. [20] have argued that soils with the highest organic carbon contents may be more resistant to stress. Specifically, SOM inputs can increase soil basal respiration [17] and microbial diversity [16] . SOM inputs can also increase the stability of soil microbial communities during disturbances [21] , even though the inputs may not increase the soil’s microbial biomass [22] . It is through such mechanisms that land use can have legacy effects on the stability of microbial biomass.
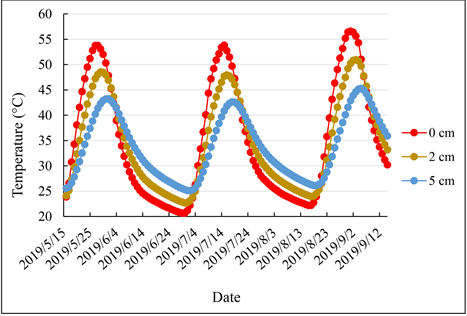
Land use in Senegal’s groundnut basin is characterized by two main practices regarding SOM management. Homefields near the homestead received a continuous application of the household’s organic waste and receive substantial amounts (ranging from 4 to 20 t·ha−1 of dry matter) of cattle and small ruminant manures every year or two [23] . Therefore, homefields are more fertile than the outfields, which receive organic fertilization ≤ 1 Mg ha−1·yr−1, and thus remain poor in organic matter and nutrients [23] [24] . The climate-related responses of soil microbial communities in the two types of fields are important to understand because of the prolonged drought that the groundnut basin has suffered during the last two decades. Rainfall has decreased [25] , with rainy breaks that frequently exceed 15 days [26] , the air temperatures sometimes reach 43˚C [27] , and between 50˚C and 60˚C at the surface of the soil in case of extreme heat (Supplementary material 1). It is known that extreme episodes of precipitation and temperature affect microorganisms in ways that progressively decelerate the decomposition of organic matter [28] . However, agriculture can adapt to climate change by adopting farm management practices that minimize the adverse effects of extreme weather conditions [29] , or that enhance soil functional stability [29] and increase sequestration of soil organic carbon (SOC) [24] [30] . Thus, understanding the effects of heat disturbances upon the stability of soil microorganisms is important for deciphering the impact of agricultural practices that farmers in the groundnut basin adopt in face of climate change. This study should allow us to quantify the resistance and resilience of soil microbial heterotrophic respiration to artificial disturbance by extreme heat [1] . Several studies used long-term experiments, exposing the soils, in laboratory or the field conditions, to increased temperatures ranging from a few degrees (25˚C) [17] to several tens of degrees (50˚ to 500˚C) [1] [31] [32] [33] [34] .
The aim of this study was to determine 1) how the duration of heat stress affects the stability of soil microbial community activity and diversity, and 2) how this response can be influenced by organic amendments. We hypothesized that 1) the effect of heat stress on the stability of microorganisms depends upon the duration of the disturbance; 2) microbial communities in fields that receive organic amendments regularly (i.e. homefields) are more resilient to heat stress than communities in outfields, which are amended less frequently; and 3) heat stress reduces the taxonomic diversity of soil microbial communities.
2. Materials and Methods
2.1. Sampling Site
Soils samples were collected from homefields and outfields in the village of Diohine, which is located in the Senegalese Groundnut Basin at 14˚29'51"N, 16˚30'36"W [23] . The local soil is classified as Arenosol [35] , with mostly low clay contents (<5%; mainly kaolinite) [24] . The climate is Sudano-Sahelian, characterized by a long dry season (October to June) and three months of rainy season from July to September. Annual rainfall is 530 mm, and the average annual temperature is 30˚C. Woody vegetation is dominated by Faidherbia albida distributed in parkland; the geological substratum consists of tertiary sandstones [24] . The homefields and outfields whose soils we sampled produced millet (Pennisetum typhoides): the area’s main crop, which in some cases is rotated with maize, cowpea, and groundnut.
2.2. Soils
Two types of plots were used (homefields and outfields) that were representative of the two cultural practices in groundnut basin. Homefields received substantial amounts of cattle and small ruminant manures every year or two ranging 4 to 20 t·ha−1 of dry matter while outfields received organic fertilization ≤ 1 Mg ha−1·yr−1 [23] .
The soil samples were collected during the dry season at depth of 0 - 10 cm in six plots of homefields and six plots of outfields (Supplementary material 2). As shown in Supplementary material 3, the homefields soils contain 0.9% clay, 2.2% silt, 96.9% sand, 0.45% SOM, 0.26% total C, 1.88 meq/100g CEC, 0.02% total N, and 4.84 ppm assimilable P. Their C/N ratio is 10.9 and the pH 6.72. The outfields soils contain 1.3% clay, 1% silt, 97.7% sand, 0.22% SOM, 0.13% total C, 1.30 meq/100g CEC, 0.01% total N, and 3.75 ppm assimilable P, with a C/N ratio of 12.9 and pH 5.86. Soils’ physico-chemical characteristics were performed at ISRA, CNRA laboratory of Bambey, Senegal (https://www.isra.sn). For the characterization of SOM and C the modified Walkley and Black [36] , method was used. The modified Olsen method was used for assimilable P, and Kjeldahl method for total N. The Robinson’s pipette method with USDA classification was used for soil texture, the ammonium acetate for CEC, and a laboratory pH-meter with electrode and extractor for the pH.
After the physico-chemical analysis, the six replicates of each practice were, pooled, sieved to <2 mm and stored at room temperature pending processing. The sample for each site consisted of six pooled subsamples.
2.3. Stress Strategy and Soil Incubation
The experiment was carried out under glasshouse controlled conditions. In the region of interest in Senegal CP4-Africa simulations (AMMA-CATCH, 2018) (Supplementary material 1), showed that temperature can exceed 50˚C at surface of the soil. Therefore, we chose 60˚C as the extreme heat-stress temperature so that we could determine which of the microbial communities is most resistant to extreme thermal stress. Further support for choosing 60˚C is found in Riah-Anglet et al. [37] , who report not only that microbial communities’ activities are affected similarly by heat stress at 50˚C and 60˚C, but that 60˚C represents an extreme thermal stress [1] [37] [38] [39] .
The heat-stress experiments and subsequent analyses were done on aliquots of six homefields and six outfields soils. The aliquots for a given pooled soil sample (i.e., six pooled subsamples of homefields soil or outfields soil) were prepared as follows. First, we determined the pooled sample’s residual water content and water-holding capacity (WHC), after which we added demineralized water to raise the sample’s water content to 80% of WHC. The aliquots’ samples were then pre-incubated at 28˚C for seven days to stabilize their microbial activity, per the recommendations of Wada and Toyota [21] .
After pre-incubation, the microcosms were made of 330 ml glass bottles filled with 30 g of equivalent dry aliquot soils at 80% WHC [6] . Each bottle was then sealed with a waterproof rubber plug. Heat stress in sealed bottles does not resemble actual heat stress in the field, but several studies have used this method to elucidate the different responses of the microbial community to disturbance and thus give trends of potential change in the real environment [1] [6] [40] [41] . We simulated extreme heat stress under controlled conditions and applied two distinct treatments: control (without stress) and heat stress. As a control sample (i.e., not heat-stressed), and following the protocols of Wada and Toyota [21] , one of the bottles was then held at 28˚C while the others were held for (variously) 3, 14, or 28 days at 60˚C to simulate heat stress [1] [37] [38] . The three durations are denoted as SD3, SD14, and SD28, respectively. We ran three replications of each duration for each pooled soil sample. After each heat-stress sample had completed its time at 60˚C, it was given a 28-day recovery incubation at 28˚C [42] .
Each bottle was weighed every 3 days, from the beginning of the heat stress until the end of the post-stress recovery incubation. Demineralized water was added as needed to maintain the soil moisture content between 70% and 80% of WHC. At the end of incubation, we took triplicate soil samples from each bottle for molecular analyses, then stored the 3 samples at -80˚C until DNA extraction.
2.4. Microbial CO2 Respiration
To quantify the metabolic activities of soil microorganisms after the heat stress [43] , we measured basal respiration (i.e., the CO2 emitted by soil samples) [14] [17] of the same aliquots of soil (i.e., those that were sealed in 330-ml bottles) that are described in Section 2.2. CO2 levels in the bottles were measured using gas phase micro-chromatography (μ-CPG Agilent 490, 1109602). Air was renewed frequently via an air pump to avoid accumulation. The total amounts of CO2 emitted (µg C-CO2 g−1 soil) were calculated after the 28-day recovery incubation for each of the 3 stress durations (SD3, SD14, and SD28). For each of those durations, C mineralization rates (µg C-CO2 g−1 soil·day−1) of a given soil sample were measured during the 28-day recovery incubation. The rate was also calculated immediately after heat stress.
2.5. Resistance (RS) and Resilience (RL) Indices
We calculated Orwin and Wardle’s RS and RL indices, which are generic ones that may be used for any sort of disturbance [5] to estimate the microbial communities’ resistance and resilience under heat stress.
The RS index for a given soil aliquot and heat-stress duration was calculated from C mineralization rates that were measured immediately after heat stress. That is, at the end of SD3, SD14, and SD28:
(1)
where D0 is the difference between P0 (in our case, the C mineralization rates of the heat-stressed sample immediately after heat stress) and C0 (the rate for the control samples; i.e., those which were maintained at 28˚C while the others were undergoing heat stress). Note t0 is the moment at which the rates were measured at the end of heat stress.
The resilience index, RL, was calculated from C mineralization rates that were measured at time tx, the end of the 28-day recovery incubation that followed SD3, SD14, and SD28:
(2)
Here, D0 is as above, and Dx is the difference between the C mineralization rates of the control soil (Cx) and the heat-stressed soil (Px) at the time point (tx) chosen to measure resilience. Note that the values of these two indices are bounded by −1 and +1 with a value of +1 showing that the disturbance had no effect (maximal resistance), and lower values showing stronger effects (i.e. less resistance). An index value of 0 indicates either a 100% reduction or increase in the value of the disturbed soil. Similarly, an RL of +1 indicates that Px = Cx (complete recovery after the disturbance), and lower values indicate slower recovery. An index value of 0 indicates that the disturbed soil has either not recovered at all since the disturbance ended (i.e. D0 = Dx) [5] .
2.6. DNA Extraction and Sequencing
After 28 days of recovery incubation, high-throughput sequencing was performed on SD28 of homefield and SD14 of outfield to find the microbial communities responsible of the partial resilience observed at the end of their 28-day recovery. For the homefield, three heat stress samples of SD28 and control without incubation and three heat stress samples of SD14 and control without incubation of outfield were used for DNA extraction.
Thus, total genomic DNA of each soil sample was extracted from 0.25 g of soil using the FastDNATM SPIN kit for Soil (MP Biomedicals, CA, USA), with modification of the manufacturer’s instructions [44] .
The quality and concentration of the extracted DNA was verified after electrophoresis migration on 1.5% agarose gel. High-throughput sequencing was performed at ADNID (Montferrier, France; http://www.adnid.fr) with Illumina MiSeq system (Illumina) targeting 16S rRNA gene with the 515F/806R primers set and ITS gene with the ITS3F-ITS4R primers. The sequences were deionized, and operational taxonomic units (OTU) were defined by clustering at 3% divergence (97% similarity) followed by removal of singletons and chimeras. Final OTUs were taxonomically classified using BLASTn against a curated database derived from GreenGenes and SYLVA. We then produced the final OTUs tables containing the number of sequences per sample per OTU matching the designated taxonomic classification. The whole process was conducted at ADNID (Montferrier, France; http://www.adnid.fr)
2.7. Statistical Analyses
The statistical analyses were performed with R software v. 3.1.3 (Peter Dalgaard, CET 2015). Normal distribution of residuals and homogeneity of variance were assessed by (respectively) the Shapiro and Bartlett tests. If these two conditions were met, one-way ANOVA was performed to analyze the effect of heat stress. A Tukey HSD test was used for pairwise multiple comparisons if heat-stressed samples and control samples differed significantly in their physico-chemical characteristics, C mineralization, or microbial-diversity indices. A Kruskal Wallis non-parametric test was performed whenever residuals were not normally distributed, or variances were inhomogeneous. Each sample’s α-diversity of bacterial and fungi communities was evaluated by calculating richness and the Shannon and Simpson indexes.
3. Results
For all statistical analyses, the level of significance is p < 0.05.
3.1. Cumulative Respiration of C-CO2
CO2 accumulation (µg C-CO2 g−1 soil) was calculated after 28 days of recovery incubation. For all 3 heat-stress durations, C mineralization in homefield samples was significantly higher than that of the controls (by 32.19% for SD3, 86.94% for SD14, and 94.12% for SD28) (Table 1). In the heat-stressed outfield samples, the C mineralization was again higher than in the controls (by 2.53% for SD3; 18.3% for SD14; and 8.96% for SD28), but only the SD14 sample’s increase was significant. C mineralization in the control homefield samples is also 2.26 to 2.5 fold the C mineralization in the control outfield samples, comparable to organic C ratio between homefield and outfield (2.69).
3.2. Organic Carbon (C) Mineralization
Heat-stressed homefield soils had high mineralization rates compared to control. The average rate for SD3 was 8.83 µg C-CO2 g−1 soil·day−1, versus 6.96 and 5.36
![]()
Table 1. Cumulative basal respiration (µg C-CO2 g−1 soil) at the end of the 28-day incubation period for SD3, SD14, and SD28 samples of homefield and outfield soils.
Superscripts indicate significant differences between stress and control samples (p < 0.05), n = 3.
for SD14 and SD28, respectively. All 3 of these rates were significantly higher (p < 0.05) than the low, stable rates of the corresponding control samples (2.22, 1.84, and 1.59 µg C-CO2 g−1 soil·day−1 respectively for SD3, SD14, and SD28) (Table 2). However, mineralization rates decreased towards the end of the heat stress and during the recovery incubation (Figures 1(a)-(c)). At the beginning of that recovery, the SD3 samples showed a respiration pulse 115.4% higher (significant at p < 0.05) than that of control samples (Figure 1(a)). In contrast, the SD14 and SD28 treatments showed no such difference in respiration flux between control and heat-stress samples (Figure 1(b), Figure 1(c)).
In the case of outfield soils, the average mineralization rates of heat-stress samples were again higher than the low, stable rates of the controls (1.44, 0.85, and 0.81 C-CO2 g−1 soil·day−1 for the heat-stressed SD3, SD14, and SD28 samples, versus 1.22, 0.94 and 0.84 µg C-CO2 g−1 soil·day−1 for the respective control samples). However, none of the differences are significant (Table 2). C mineralization rates decreased towards the end of the heat stresses and at during the recovery incubation (Figures 2(a)-(c)). In addition, the respiration pulses exhibited by heat-stress samples at the beginning of the recovery incubation were higher than those of the corresponding controls (157.9% higher for SD3, versus 302% and 75.5% respectively for SD14 and SD28) (Figures 2(a)-(c)).
3.3. Effect of Heat Stress upon Carbon Mineralization
The carbon mineralization analyses were performed at the end of heat stresses (t0) and at the end of the 28-day recovery incubations (t28). The resulting mineralization rates were compared to those for control samples, via appropriate statistical analyses.
3.3.1. Effect upon Resistance
In the homefield samples, the SD3 and SD14 heat stresses did not affect C mineralization. The total amount of C mineralized was not significantly different between heat-stressed and control samples at the end of SD3 and SD14. However, for SD28 the C mineralization of the control samples was significantly higher (97.87%) than that of the heat-stressed soil. This result indicates an effect of heat stress (Table 3).
In the outfield samples, C mineralization was disrupted by SD3, SD14, and SD28 heat stresses. Thus, the total amount of C mineralized was significantly different between the heat-stressed and control samples in all three treatments. At the end of SD3 heat stress, the C mineralization rate of the heat-stressed sample was 83.08% higher than that of the control. However, at the end of SD14 and SD28 heat stress, the C mineralization rates of control samples were respectively 84.09% and 70.97% higher than those of the heat-stressed samples (Table 3).
![]()
Figure 1. C mineralization rates (C-CO2 kg−1 soil·day−1) in the homefield for the three heat-stress durations SD3 (a), SD14 (b) and SD28 (c). Negative values (−30 to 0) on the x-axis represent heat stress (60˚C) and control (28˚C). Positive values (0 to 30) on the x-axis refer to recovery days (28˚C). The dotted lines separate the heat stress and the recovery phase. Blue curve: control; red curve: heat stress.
![]()
Table 2. Average of C mineralization (µg C-CO2 g−1 soil) for the control and heat-stressed SD3, SD14, and SD28 samples of homefield and outfield soils during the heat stress and the recovery days.
Superscripts indicate significant differences between stress and control samples (p < 0.05). n = 3.
![]()
Table 3. Rates of C mineralization (µg C-CO2 g−1 soil·day−1) for the control and heat-stressed SD3. SD14. and SD28 samples of homefield and outfield soils. Resistance (RS) and resilience (RL) indices are based on C mineralization rates for SD3. SD14. and SD28 of homefield and outfield samples.
Superscripts indicate significant differences between heat-stress and control treatments (p < 0.05). n = 3.
![]()
Figure 2. C mineralization rates (C-CO2 kg−1 soil·day−1) in the outfield for the three heat-stress durations SD3 (a), SD14 (b), SD28 (c). Negative values (−30 to 0) on the x-axis represent heat stress (60˚C) and control (28˚C). Positive values (0 to 30) on the x-axis refer to recovery days (28˚C). The dotted lines separate the heat stress and the recovery phase. Blue curve: control; red curve: heat stress.
3.3.2. Effect upon Resilience
In the case of homefield samples, C rates in heat-stressed samples remained significantly lower (p < 0.05) than those of the control samples even after the 28-day recovery incubation. Specifically, the mineralization rate in the control soil was 36.23% higher for SD3, versus 26.21% higher for SD14 and 35.59% higher for SD28 (Table 3).
In the case of outfield soils, the C mineralization of the heat-stressed SD14 sample was not significantly different, after the 28-day incubation, from the control’s rate. This result indicates a recovery of C mineralization. In contrast, the mineralization rates of the heat-stressed SD3 and SD28 samples were significantly different (again, after the 28-day incubation) from those of the control samples. Specifically, the control-sample’s rate was 40.54% for SD3, and 66.67% higher for SD28 (Table 3).
3.4. Resistance (RS) and Resilience (RL) Indices of C-CO2 Respiration
These indices, proposed by Orwin and Wardle [5] , are generic ones that can be calculated from different types of data to highlight moderate effects upon function that might not be revealed by simple statistical analyses. We calculated these indices from C-mineralization results, as described in Section 2.4.
3.4.1. Resistance
In the homefield soils, the RS indices for the SD3 and SD14 heat stresses were 0.70 and 0.80 respectively. These high values indicate that the microbial communities were not affected significantly by the heat stress. However, the RS value for SD28 was 0.01, indicating an effect of heat stress (Table 3).
In the outfield soil, the RS indices for SD3, SD14, and SD28 were respectively 0.10, 0.08, and 0.17. Those low values indicate an effect of heat stress whatever the stress duration considered (Table 3).
3.4.2. Resilience
In the homefield soils, the RL indices for SD3 and SD14 were low and negative (−0.31 and −0.26 respectively), indicating a lower rate of recovery than in the SD28 sample, for which RL was 0.63. The latter high, positive value indicates a progressive recovery of C mineralization (Table 3). In the outfield soils, the RL indices of SD3 and SD14 were high (0.61 and 0.76 respectively), indicating a progressive recovery, in contrast to the lower recovery rate that can be inferred from the SD28 sample’s low, negative value (−0.13) (Table 3).
3.5. α-Diversities
Using >97% sequence identity, the high-quality reads were clustered into operational taxonomic units (OTUs). The bacterial and fungal α-diversities of homefield soils and outfield soils (as quantified by richness and the Shannon and Simpson indices) were quite different (Table 4).
In the homefield soils, bacterial OTU richness was significantly greater in the heat-stressed soils than in the control soils. In contrast, fungal OTU richness was significantly greater in the controls. In the outfield soils, OTU richness was significantly greater in the control soils for the bacteria as well as the fungi. These results indicate that in the homefield soils, heat stress increased the bacterial richness, but decreased the richness of fungi, whereas heat stress reduced the richness of both types of microbes in outfield soils.
![]()
Table 4. Bacterial and fungal diversity indices for SD28 of homefield and SD14 of outfield samples under control and heat-stress treatment.
Superscripts indicate significant differences between heat-stressed and control samples according to software R (3.1.3). (p < 0.05). n = 3.
The specific diversity, as quantified by the Shannon and Simpson diversity indices, varied across the heat-stress treatments. In the homefield soils, these indices and the OTU richness showed the same trends for the bacterial community. For fungi community in the homefield soils, the Shannon and Simpson indices for heat-stressed samples were not significantly different from those of control samples. However, in the outfield soils the values of the indices for fungi were higher in control samples than in the heat-stressed ones (Table 4).
3.6. Taxonomic Composition of Bacteria and Fungi Communities
In homefield and outfield soils alike, heat stress changed the relative abundances (as compared to control samples). The taxonomic inventory of the sequences identified 14 bacterial and 7 fungal phyla. The dominant bacterial phyla (relative abundance > 1%) were Firmicutes, Proteobacteria, Chloroflexi, Actinobacteria, Acidobacteria, Bacteroidetes, Gemmatimonadetes, Planctomycetes, and WPS-2 (Figure 3(a)). The major phyla of fungi were Ascomycota, Basidiomycota, Chytridiomycota, and Mucoromycota (Figure 3(b)).
At the class level, we found thirty bacteria classes, all of which were present in every sample, and belonged mostly to the phyla Firmicutes, Chloroflexi, Proteobacteria, Acidobacteria, and Actinobacteria. In the non-stressed samples from homefield, the bacterial and fungal classes Bacteroidia, Blastocatellia, Gemmatimonadetes, Nitrososphaeria, δ-Proteobacteria, Chytridiomycetes, and Sordariomycetes were significantly more dominant than in the non-stressed samples from outfield. In the latter samples, the dominant classes were Acidobacteriia, Bacilli, Ktedonobacteria, WPS-2-unknown-class, Dothideomycetes, and Glomeromycetes (Figure 3(c), Figure 3(d))).
3.7. Changes in Microbial Population under Heat Stress
In the homefield soils, heat stress reduced the relative abundances of the following bacteria taxa significantly: Acidobacteriia (from 1.3% relative abundance to 0%); α-proteobacteria (13.5% to 1.75%), γ-proteobacteria (14.51% - 0.05%), Bacteroidia (2.66 - 0.02), Blastocatellia (2.24% - 0.5%), Thermoleophilia (2.37% - 0.29%), WPS-2 (0.2% - 0.0%). The relative abundances of two fungi taxa also decreased: Dothideomycetes (25.68% - 7.25%) and Eurotiomycetes (8.43% - 3.67%). In contrast, the relative abundances of the following taxa increased significantly: the bacteria Actinobacteria (6.11% - 18.73%), Bacilli (42.08% - 56.58%), Chloroflexia (0.82% - 19.62%), and Clostridia (0.46% - 1.70%), as well the fungi taxa Sordariomycetes (44.41% - 69.92%) and Glomeromycetes (0.02% - 1.20%) (Figure 4(a), Figure 4(b)).
![]()
Figure 3. Relative abundances (%) of OTUs of bacterial (a) and fungi (b) phyla and of bacterial (c) and fungi (d) classes in microbial communities identified in control and heat-stressed samples of homefield and the outfield soils.
![]()
Figure 4. Percentage (%) difference between bacteria and fungi community at classes (a, b) and species (c, d) levels in heat-stressed and control samples of homefield and outfield soils.
In the outfield soils, heat stress reduced the relative abundances of the following bacteria taxa significantly: Acidobacteriia (1.17% - 0.0%), Actinobacteria (10.50% - 0.11%), Chloroflexia (0.24% - 0%), Thermoleophilia (2.45% - 0%), WPS-2 (2.41% - 0%). The relative abundances of four fungi taxa also decreased: Chytridiomycetes (0.34% - 0%), Dothideomycetes (72.81% - 0.30%), Glomeromycetes (0.11% - 0%), and Sordariomycetes (8.09% - 0.27%) (Figure 4(a), Figure 4(b)). However, the relative abundances of the fungi taxa Eurotiomycetes increased significantly (13.82% - 91.84%), as did the abundances of the bacteria taxa α-Proteobacteria (10.35% - 26.74%), γ-Proteobacteria (11.10% - 14.32%), and Clostridia (0.6% - 0.78%), (Figure 4(a), Figure 4(b)). Comparisons between heat-stressed outfield soils samples and the controls found no significant change in the relative abundances of the most dominant Bacilli class (54.01% in the controls, versus 53.53% in heat-stressed samples). Bacilli in outfield soils seemed to be insensitive to heat stress (Figure 4(c), Figure 4(d)).
At the species level, we found that the following bacteria species dominated in the homefield control soils: Bacillus fumarioli (11.76%), Ammoniphilus resinae (5.81%), Microvirga sp (4.29%), and Flavisolibacter sp. (3.77%). Heat stress significantly reduced the relative abundance of Microvirga sp. and Flavisolibacter sp, but allowed a significant increase in the relative abundances of Sphaerobacter thermophilus, Aeromicrobium sp., and Cohnella sp (Figure 4(c)). The most dominant fungal species in the homefield control soils were Phaeoacremonium minimum (22.21%), Chaetomium sp. (21.49%), Pleosporales sp (8.36%), and Westerdykella cylindrica (5.20%). Heat stress reduced the relative abundances of those dominant species, but significantly increased the relative abundance of Sarocladium sp. (0.01% - 80.05%) (Figure 4(d)).
In the outfield control soils, the most dominant bacteria species were Bacillus fumarioli (24.46%), Paraburkholderia fungorum (8.33%), Tumebacillus sp. (6.90%), Arthrobacter sp. (7.28%), Ammoniphilus resinae (6.61%), and Sphingomonas echinoides (6.51%). Heat stress significantly reduced the relative abundances of Ammoniphilus resinae, Bacillus fumarioli, and Arthrobacter sp., while significantly increasing those of Tumebacillus sp., Sphingomonas echinoides, and Cohnelle sp. (Figure 4(c)). The most dominant fungal species in the outfield soils control were Boeremia exigua var. exigua (39.17%), Alternaria alternata (16.67%), Rhodotorula sp. (6.57%), and Penicillium chrysogenum (6.52%). Heat stress significantly reduced the dominant species, and allowed Aspergillus lentulus and Exophiala oligosperma to become dominant (Figure 4(d)).
4. Discussion
4.1. Differences between Properties and C-CO2 Mineralization in Homefield and Outfield Soils
Some but not all of the responses of the homefields’ microbial communities differed from those of outfields. In addition, some responses of heat-stressed soils differed from those of the controls. For example, heat-stressing of both soils produced a high biological activity that did not occur in the controls. However, the C mineralization in homefield soils was greater than in outfield soils. These results are consistent with those of Ågren and Wetterstedt [45] , who attribute them to a strong, temperature-induced C mineralization, and thus to an increase in basal respiration of the microbial community. Franco-Andreu et al. [46] explains the same results by citing mineralization of labile carbon an immediate source of energy for microorganisms.
For homefield soils as well as outfield soils, and for all heat-stress durations, C-CO2 accumulations at the end of incubation were higher than those of the controls. As one example, the accumulations (taken as a class) for homefield soils that received the SD28 heat stress exceeded those of the controls by 58.25 µg C-CO2 g−1 soil. However, accumulations for outfield soils that received SD28 exceeded those of the controls by only 2.45 C-CO2 g−1 soil. Chotte et al. [1] obtained similar results in their research on manured and non-manured soils: the C-CO2 accumulation of a heat-stressed manured soil exceeded that of the control by 68.8, µg C-CO2 g−1, versus 15.2 for the non-manured soil. That difference is explained by the high level of organic C in Chotte et al. [1] soils (20.1 and 18.3 mg·g−1 soil, for manured and unmanured soils respectively). By comparison, the organic C contents in our soils were 10.5 and 3.9 mg·g−1 soil in homefield and outfield samples, respectively.
Chotte et al. [1] work also agrees with our finding that C mineralization is significantly higher in soil amended with OM (homefield) than in the unamended (outfield). Specifically, Chotte et al. [1] found that the respiration of samples amended with OM is significantly higher than for unamended samples. Ågren and Wetterstedt [45] attribute the difference in C mineralization to temperature-induced solubilization of organic compounds.
The decrease in soil microbial respiration at the end of incubation is explained by a decrease in soil’s labile C fractions due to decomposition during heat stress [47] . Davet [48] agrees and states that biological activity decreases with resource depletion after 2 to 3 weeks at a level equal to or lower than that of the control soil. The slight recovery of microbial activity that occurred in our samples at the beginning of recovery confirms the results of Pailler [49] , who attributed it to a significant development of microbial metabolism. At the end of a disturbance (according to that author), the microbial community increases its basal respiration in order to resume metabolic activities.
4.2. Stability of the Soil Microbial Biomass
C mineralization in homefield soils that underwent SD3 and SD14 was not statistically different from that of the controls. This result suggests that the microbial biomass resisted the SD3 and SD14 heat stresses. However, and in contrast to the raw-data comparison, the RS indices that we calculated from C-mineralization data did not show complete resistance to those two durations of heat stress. Furthermore, none of the RS values for homefield and outfield show complete resistance of the microbial biomass to any of the 3 durations. These findings are similar to those of Chotte et al. [1] , who found that OM inputs to homefield did not modify the microbial community’s resistance. Ben Sassi [13] also states that OM inputs do not improve microbial stability to an important degree. In contrast, Wada and Toyota [21] , show that the resistance of biological functions is higher in the presence of OM. The discrepancy between that result and our own could be explained by the organo-mineral amendment that was used on fields that were studied by Wada and Toyota.
In our own study, microbial communities in the OM-rich soil of homefield had high resistance to SD3 and SD14 heat stresses (RS = 0.70 and 0.80 respectively for SD3 and SD14). Therefore, those durations of heat stress do not disrupt all biological activities in the homefield samples. In contrast, RS indices for outfield soils were low for all three heat-stress durations. These results are similar to those of Griffiths et al. [50] , who found thermal-stress resistance in soils amended with OM. However, our results show that the SD28 heat stress did disrupt microbial activity of homefield soils (RS = 0.01). Thus, the response of microorganisms to heat stress depends upon the duration of the stress [13] [14] .
The resilience indices (RL) of homefield and outfield soils show no recovery of the microbial biomass for any of the 3 durations of heat stress. This result is similar to those of Fierer and Schimel [51] , who observed no resilience after 6 weeks of recovery. The absence of complete resilience may be due to insufficient recovery time. For example, Kumar et al. [42] found that microorganisms needed 56 days to recover fully. Too, the extreme temperature of 60˚C can impede recovery by removing a large proportion of the active microorganisms from the soil [12] .
However, in the present study the RL indices do show a beginning of resilience in outfield soils after SD3 (RL = 0.56) and SD14 (RL = 0.76), and in homefield soils after SD28 (RL = 0.63). Similarly Bérard et al. [31] found no complete resilience of microbial communities at the end of recovery. The partial recovery after SD28 in homefield soils (versus the non-recovery in outfield soils) is explained by the organic amendment. Indeed, the soils richest in C are more resilient in response to intense heat stress [20] [21] [52] .
4.3. Microbial Biomass Diversity and Abundance
In our nutrient-poor outfield soils, heat stress not only decreased the microbial α-diversity significantly, but altered the abundances of most of the microbial taxa. However, only the fungal diversity decreased in the homefield soils. As will be explained below, these differences between the two soils may be attributable to several phenomena that interact in subtle ways. Those phenomena include the effects of heat upon the soils’ labile C contents, as well as the ranges of metabolic and functional flexibility within the two soils’ microbial communities.
In both soils, fungal and bacterial diversity was lower in the heat-stressed samples than in the controls. The decrease was greater in outfield soils. That result is consistent with Bécaert et al.’s [38] observation that heat stress can kill microbial communities, and that the impact tends to be greater in lower-OM soils. The high microbial diversity in the homefield soils is related to the legacy effect of OM, a substance that microorganisms depend upon to support their activity [8] .
In our study, OM’s legacy effect manifested itself in differences between the changes in taxonomic compositions of the two soils’ respective microbial communities. Heat stress reduced the relative abundances of homefield microbial communities that belong to α-proteobacteria, γ-proteobacteria, Bacteroidia, and Eurotiomycetes. In contrast, heat stress increased the abundances of those same microbes in outfield soils. One explanation for the decreased abundances in homefield soils may be that soil’s C resources diminished during heat stress, thereby exacerbating the vulnerability of the copiotrophic communities that normally proliferate in such resource-rich soils [53] [54] . The effect of heat stress upon C resources may also explain the outfield soils’ increased abundances of copiotrophs: heat stress probably promoted decomposition of recalcitrant SOM, thereby enriching soils with labile C. The increased abundance of copiotrophs in heat-stressed outfield soils is consistent with Davet [48] , finding that some species of Eurotiomycetes can grow at temperatures up to 60˚C [55] [56] .
An interesting contrast to that finding is offered by the relative abundances of Actinobacteria, Chloroflexia, and Sordariomycetes, which are highly stress-tolerant [57] . In the homefield soils, heat stress increased the abundances of these communities, perhaps by reducing competition from heat-sensitive microbes. In addition, the metabolic versatility of Actinobacteria, Chloroflexia, and Sordariomycetes enables them to develop in soils where recalcitrant carbon prevails [58] [59] [60] . For example, the Chloroflexia class is dominated by Sphaerobacter thermophilus species, whose optimal growth temperature is 55˚C - 60˚C [61] .
The above-mentioned metabolic versatility of microbes must be borne in mind when interpreting our RL indices, which were calculated from C-mineralization data (a measure of the microbial community’s functioning, rather than its taxonomic composition). As Preece et al. [62] notes, a partial resilience such as that which occurred in both of our soils may accrue from the microbial communities’ capacities for functional redundancy. Similarly, Riah-Anglet et al. [37] , found that heat stress does not affect bacterial abundances in soils that are rich in Actinobacteria and Bacteroidetes. For example, the variable and versatile physiology of Proteobacteria gives them a competitive advantage in various ecological niches [63] . As the continuing heat stress reduces competition from other species for soil resources [37] , new stress-resistant microbial communities with greater resilience and functional stability can develop [64] . In the event of a disturbance, these communities may either develop adaptation strategies (resistance) [65] , or remain inactive while waiting for conditions to become favourable (resilience) [66] .
In our study, the representatives from the most dominant bacterial class (Bacilli) seemed to be insensitive to heat stress, as evidenced by the fact that the relative abundance of that class did not decrease in the outfield soils. Nor did heat stress change the taxonomic composition of that class in outfield soils. Allison and Martiny [67] , define a microbial community’s composition as resistant if that composition is difficult to perturb. That resistance is enhanced by high degrees of metabolic flexibility and physiological tolerance to changing environmental conditions Allison and Martiny [67] . The heat-stress resistance of Bacilli in our study is consistent with previous studies stresses [53] [60] [68] that posit the production of heat-resistant endospores by Bacillus species as a means of resisting environmental stresses. As Kämpfer et al. [69] noted the Bacilli class is dominated by Cohnella sp, Lysinibacillus macrolides, Aeromicrobium sp., and Sphingomonas echinoides, which are able to grow at temperatures between 20 and 55˚C. In our present study, the Actinobacteria, Chloroflexia, and Sordariomycetes taxa (in homefield) and the Bacilli, α-proteobacteria, γ-proteobacteria, and Eurotiomycetes (in outfield) demonstrated their ability to resist heat stress via different strategies that members of those taxa employ to resist environmental stresses, and adapt to them.
5. Conclusions
This study focused on the effect of heat-stress duration upon the stability (resistance and resilience) and diversity of microbial communities in fields that received different amounts and types of organic amendments. The responses of microbial community composition to heat stress varied according to agricultural practices and the duration of the stress. Communities were not stable in either soil, and microbial α-diversity decreased at different heat stress durations. Communities in the OM-rich homefield soils showed a partial resistance to shorter-duration stresses (SD3 and SD14), and also showed the beginnings of resilience even after the longest stress (SD28). In contrast, microbial communities in the low-OM outfield soils displayed no resistance to any of the three heat-stresses durations, although the beginnings of resilience were noted after SD3 and SD14.
Even in the OM-rich outfield soils, the communities did not show total resistance to 60˚C, nor was resilience complete after the 28-day recovery. Nevertheless, soil OM does appear to increase the resilience of the microbial community composition in the face of long-duration heat stress. The same heat stress that decreased the microbial diversity also brought about microbial communities that are specific to each farming practice, and which could contribute to the resilience and/or resistance of the respective soils.
Future research should focus on the effect of drought and drought-heat stress on the resilience of soil microbial communities in the outfields and homefields cultivation practices of the groundnut basin.
Funding
The research leading to this study received funding from the U.K.’s Natural Environment Research Council/Department for International Development (NERC/ DFID) Future Climate for Africa (FCFA) program, under the AMMA-2050 (Grant Numbers NE/M020126/1).
Acknowledgements
This work is dedicated to Prof. Mariama Dalanda Diallo who took part in it and left us too soon. The authors are thankful to Laurent Cournac and Lydie Lardy (IRD), Cheikh Oumar BA (IPAR), Yacine Ndour-Badiane (LNRPV/FAO), Lamine Sagna, Mayecor Diouf, Moutapha Sané, Oumar Faye, Mariama Gueye, Lamine Dieng, the late Amadou DIOP and Pourmera GASSAMA (LEMSAT) and all the staff of LMI IESOL, IPAR and ISRA/LNRPV for their technical, financial and administrative support during this research. English language was editing and review services supplied by James Smith (nitac14b@yahoo.com).
Supplementary Material 1. Temperature of the Soil at the Surface Horizon (0 - 5 cm) in the Groundnut Basin Using CP4-Africa Simulations (AMMA-CATCH) [70] [71]
Supplementary Material 2. Geographical Coordinates of Six Homefields and Six Outfields Individual Sampled
Supplementary Material 3. Physico-Chemical Characteristics of Homefields and Outfields Soils
Superscripts indicate significant differences between homefields and outfields (p < 0.05) n = 6, CEC: cationic exchange capacity; Pass: assimilable phosphorus; N: nitrogen; C: carbon; SOM: Soil Organic Matter.