Heritability and Genetic Correlation of Niamey’s Local Chicken Growth (Niger) ()
1. Introduction
Local chicken makes up 57% of Niger’s poultry population [1]. Due to its availability and accessibility, it is one of the main sources of animal protein. It can be found in almost every rural Nigerien household and its meat is less expensive than that of large livestock [2]. However, this local chicken is not very productive [3] [4] and its performance needs to be improved in order to combat poverty and food insecurity.
Industrial strains are potentially more productive, but the Sahelian climate of Niger, characterized by high temperatures and low humidity, can lead to a decrease in performance and increased production costs [5]. In these industrial strains, heat stress can cause a decrease in food intake [6] and an increase in the allocation of food energy to thermoregulation rather than growth [7] [8]. In addition, behavioral disturbances such as cannibalism and heat stroke can lead to high mortality [5] [8]. On the other hand, local chickens, despite their lower productivity levels, are well adapted to local climatic conditions [4]. One way to improve their performance would be through the implementation of genetic improvement systems [9].
The improvement of performance through selection requires first of all the knowledge of certain genetic parameters such as heritability and genetic correlations between traits to be improved [10]. Indeed, the knowledge and the consideration of these parameters allow to estimate the expected genetic gain and to better define the strategies to be implemented [11].
Two statistical approaches (Frequentist and Bayesian) can be used for the estimation of genetic parameters. For the frequentist approach, the probabilities represent the frequency of events after a large number of repetitions of an observation or experiment, whereas the Bayesian approach interprets these probabilities as our uncertainty about the value of a quantity [12] [13]. Therefore, contrary to the Bayesian approach, the frequentist approach requires a high number of observations to obtain a reliable estimate of the parameters. Indeed, in a classical (frequentist) approach, a reliable estimation of heritability or genetic correlations would require a sample size of at least 1000 subjects [14]. On the other hand, as Robert (2006) [15] states “...an a priori model is certainly important for small samples, but it is also less and less important as the sample size increases...”
However, the use of the Bayesian approach imposes the choice of an a priori distribution whose adequacy with the data conditions the reliability of the final estimates [16]. Thus, if the data are normally distributed, it is advisable to use an inverse Wishart distribution because it is the conjugate distribution for the covariance matrix of a multivariate normal distribution [17].
The choice of the parameters of this prior distribution is also important. Using the MCMC algorithm, it is recommended to test several versions of the prior distribution by modulating its variance (nu). And the a priori version that should be retained for the final execution of the model will be the one for which the sample size of the a posteriori distribution will be the highest and the autocorrelations lower and whose deviance information criterion (DIC) is the largest [18].
The objective of this study is to estimate, using the Bayesian method, the genetic correlations between the weights of chickens at birth (P0), 4 weeks (P4), 8 weeks (P8), 12 weeks (P12), 16 weeks (P16) and 20 weeks (P20) and the heritabilities of these weights.
2. Materials and Methods
2.1. Animals
Eggs from local salmon-gold hens were incubated to produce 14 roosters and 55 hens. Each rooster was raised with 3 - 4 hens in separate cages (14 breeding groups). The eggs produced by each group were incubated separately. The 14 offspring groups consisted of 119 chickens of which 58 were females and 61 were males. Table 1 gives the distribution of the number of offspring per breeding group.
2.2. Data Collection
The data collected were mainly weights at hatch (P0), 4 weeks (P4), 8 weeks (P8), 12 weeks (P12), 16 weeks (P16) and 20 weeks (P20). Measurements were made using a digital balance with an accuracy of 1 g.
2.3. Data Analysis
All statistical analyses were performed with the RStudio interface of the R software [19] [20]. A multivariate animal model was used to estimate genetic correlations and heritabilities using the MCMCglmm package [21] [22] with sex (male or female) and generation (parent or offspring) as fixed effects. The weights were standardized (centered-reduced values around their means) in order to minimize variance differences between weights at different ages and to facilitate the convergence of the chains of the MCMC algorithm [23].
2.4. Model Running
Details of the model used are given in Annex A1. Three variants of the Inverse-Wishart prior distribution were used to run the model (Appendix). The purpose was to assess the effect of varying priors on the model results. The variant selected to finally run the model was the one with the highest sample sizes of the posterior distributions and the lowest autocorrelations and with the largest DIC (deviance information criterion). This is the inverse-Wishart prior: V = diag(6), nu = 6 (modified inverse-Wishart).
![]()
Table 1. Distribution of the number of offspring by breeding groups.
For all the variants of priors, the MCMC algorithm has been run for a total number of 1,000,000 iterations, the registration of the samples of the a posteriori distribution has been done at each 100 iterations. The beginning of the recordings was from 3000 iterations in order to minimize the autocorrelations between samples [21] [23].
3. Results
3.1. Weight Evolution
The evolution of the weights of founders (F0) and offspring (F1) is recorded in Table 2. At hatching, the weights of the two groups varied from 23 to 25 g with little dispersion around the mean as shown by the standard error values.
3.2. Heritability
Table 3 shows the variance components as well as the heritability of weights at different ages. The estimated heritability for hatching weight and 8, 12, 16, and 20 weeks of age were high. Only the heritability of weight at 4 weeks of age was moderate.
![]()
Table 2. Means (m) ± standard error (se) in grams of chicken weights according to generation (founders and offspring), age (0, 4, 8, 12, 16 and 20 weeks) and sex.
![]()
Table 3. Estimates and credibility intervals [CI] of additive variance components; phenotypic variances and weight heritabilities at different ages.
3.3. Genetic Correlations
The estimated genetic correlations between the different weight measures are recorded in Table 4. All genetic correlations were positive. The strongest correlations were observed between weights at ages ranging from 8 to 12 weeks. Hatching weight (P0) was weakly correlated with all other weights. With the exception of hatching weight, all other weights had strong correlations with 8-week weight.
4. Discussion
4.1. Heritability
Similar heritability values to the present study have been reported for Thai Betong (KU Line) local chicken, Egyptian local chicken (Matrouh, Mandarah, Inshas and Monzatah Silver) and Iraqi local chicken [24] [25] [26]. However, lower values, ranging from 0.15 to 0.25 have also been reported for Egyptian Horro chicken and in Nigeria [27] [28]. In general, heritability values for poultry growth traits are moderate to very high [14]. This may, in part, explain the significant improvements in growth performance achieved in this species through genetic selection [11]. Other factors that have contributed to and accelerated this genetic gain are the small size of the animals, allowing thousands of animals to be raised in the same environment, and especially their prolificity coupled with a short reproductive cycle [29]. But all this would not have been possible without the consequent contribution of the fields of health and nutrition [30]. Although a high heritability value predicts a rapid response to selection, this value refers only to the group of animals on which it has been estimated in relation to their environment [31]. Thus, although the heritabilities estimated in this study indicate that nearly 50% of the observed variability is genetic in origin, the improvement of these traits by selection will also be conditioned by environmental factors.
![]()
Table 4. Estimates and credibility intervals for estimated genetic correlations between weights at hatching (P0), 4 weeks (P4), 8 weeks (P8), 12 weeks (P12), 16 weeks (P16) and 20 weeks (P20).
4.2. Genetic Correlations
The estimates of genetic correlations in this study are consistent with those reported by other authors on the same parameter in local chickens [25] [26] [27] [32]. For all these studies, and as with our results, only hatch weight is weakly correlated with the other weights. The strong genetic correlations between 8-week weight and 12-, 16-, and 20-week weights indicate that a genetic improvement in 8-week weight would also result in improved weights at 12, 16, and 20 weeks. It can also be speculated that egg weight at laying age (20 weeks) could be improved by selecting larger females as Beaumont et al., (2011) [33] state that egg weight at laying age is positively correlated with pullet weight at laying age. Also, a selection from 8 weeks of age would reduce the production costs due to the management of the farm (feeding, health) because only the selected birds will be raised beyond 8 weeks of age.
4.3. Limitations and Perspectives
Knowledge of the genetic parameters of these chickens is only part of the solution to improve their productivity. The feeding and health aspects are also very important factors in obtaining these results. Indeed, it was necessary to fix all these environmental factors to ensure the reliability of the estimates of these genetic parameters. Considering that these animals have feed conversion ratios ranging from 3.38 to 3.45 [9], the investments attributable to quality feeding and health monitoring can be costly and economically inefficient. Especially since the availability and accessibility of food is a real problem in the West African sub-region and in Niger in particular [34] [35]. Consequently, improving the productivity of this local poultry should be based primarily on reducing feed costs. This can be done by first breeding them with a standard strain with better feed efficiency and then continuing the selection process while using non-conventional feed resources to avoid competition with human populations.
5. Conclusion
Our results indicate that local chickens in Niger can respond effectively to genetic selection for live weight improvement. But also, it is possible to reduce production costs by opting for a selection at 8 weeks. Indeed, the reduction of the number of animals through the exclusion of those that do not meet the selection criteria at this age would reduce the resources related to the management of the farm beyond this age. However, it should be noted that these estimated values only refer to this group of birds and that these parameters may change over time and according to the environment.
Acknowledgements
Authors thank Dr. Ahmet Moustapha, veterinarian, and temporary worker at the Faculty of Agronomy of the University of Niamey for his help in health monitoring.
Funding
This study is funded by the Belgian Academy of Research and Higher Education (ARES) as part of the research and development project: Improvement of the poultry sector in the Niamey region (AFARNi).
Author’s Contributions
Conceptualization: A.G.T.; data curation: A.G.T.; funding acquisition: J.D. and C.M.; methodology: A.G.T., J.D. and S.I.; supervision: N.M., J.D. and S.I.; writing—original draft: A.G.T.; review and editing: N.M., S.I., J.D. and C.M.
All authors have read and agreed to the published version of the manuscript.
Appendixes
A.1. Multivariate Model Details
Equation (1) represents the multivariate model used to estimate the genetic correlations and heritability. Equations (2) and (3) represent respectively the Expectation and the Variance of the model.
(1)
where
y1 to y6 are the phenotypic values of the 6 traits (P0; P4; P8; P12; P16 and P20);
X1 to X6 are the impact matrices of the fixed effects of the 6 traits;
Z1 to Z6 are the impact matrices of the random effects of the 6 characters;
b1 to b6 are the vectors of the fixed effects of the 6 characters;
a1 to a6 are the vectors of additive genetic effects of the 6 traits [a ~ N(0,
)];
e1 to e6 are the vectors of the residual effects of the 6 traits [e ~ N(0,
].
The expectation and variance of the model are obtained as follows:
(2)
and
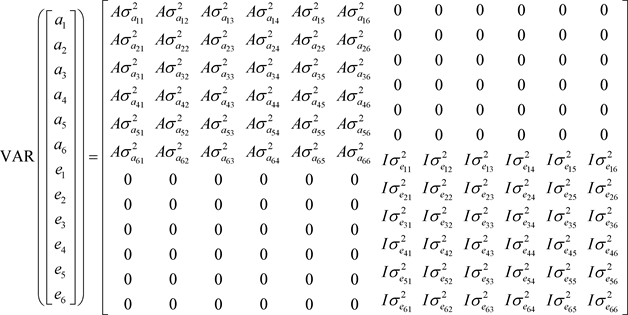 (3)
(3)
where
A: corresponds to the matrix of additive genetic relationships resulting from the pedigree;
to
are additive genetic (co)variances;
to
are the residual (co)variances and I is the identity matrix.
A.2. Tested Priors
The priors that were tested are the following:
prior1 (Invers-Wishart): V = diag(6); nu = 1.002
prior2 (Invers-Wishart modified 1): V = diag(6); nu = 1.02
Prior3 (Invers-Wishart modified 2): V = diag(6); nu = 6
A.3. Model Convergence Diagnosis
In the case of the multivariate model, the “autocorr.diag” and “effectiveSize” functions provide the autocorrelations and sample sizes by combining the variables two by two. As a result, the values reported in Table A1 6 are the highest autocorrelations and smallest sample sizes recorded between any two of the variables. Prior 3 has the lowest autocorrelations and effectiveSize similar to the other two priors.
![]()
Table A1. Autocorrelations and sample sizes by priors tested.
A.4. Effects of Priors on Estimated Heritability and Its Components Values
Table A2 shows the variance components and heritabilities for the three priors tested. The additive and phenotypic variance values as well as the heritabilities resulting from the use of the 3 priors differ little.
Prior 3 is chosen for the final multivariate model.
![]()
Table A2. Additive variances; phenotypic variances and heritabilities of weights at different ages according to the priors.
Va: Variance additive; Vp: Phenotypic variance and [CI]: [Credibility interval].