Diffuse Cervico-Facial Cellulitis: Epidemiological, Diagnostic and Therapeutic Aspects at the Teaching Hospital CNHU HKM of Cotonou ()
1. Introduction
Diffuse cervico-facial cellulitis (CCF) or diffuse fasciitis is an inflammation of the cellulo-adipose tissues of the neck and face, often of a polymicrobial nature with an extensive tendency by propagation along the various cervical aponeuroses [1]. These are polymicrobial infections involving aerobic and anaerobic germs [2] [3]. The portal of entry is most often located in the bucco-dental or pharyngeal region, with direct contamination of the cervical region by transmucosal route, unlike infections by lymphatic or hematogenous spread [3]. True diffuse fasciitis is to be differentiated from diffuse cellulitis. The latter are initially circumscribed cellulitis, which have spread to the neighboring facial or even cervical compartments. The latter lack the rapidly extensive necrosis component [2]. Treatment combines [3] [4] antibiotic therapy, drainage of the purulent collection and treatment of the cause [5]. In this study, we took into account both diffuse cellulite and true diffuse cellulite. CCF is associated with potentially significant morbidity and mortality, and it always remains a medical-surgical emergency [3] [4]. In view of the morbidity and mortality linked to this pathology, [5] [6] [7], we proposed to study its epidemiological, diagnostic and therapeutic aspects in a hospital environment in our practice context.
2. Material and Method
The study was descriptive, retrospective and took place from January 1st, 2018 to December 31st, 2020. All the patients who consulted in the ENT-CCF department at the National Teaching Hospital of Cotonou during the study period, and in whom the diagnosis of diffuse cervico-facial cellulitis was retained then the therapeutic management carried out. Diffuse cervico-facial cellulitis was evoked on clinical grounds. It was a diffuse, sensitive, hot cervico-facial swelling with a taut, shiny, dark, fissured or necrotic skin covering. Suffusion of pus and evidence of necrotic subcutaneous tissue were characteristic. Were excluded, Cases of circumscribed cervical or facial cellulitis, as well as incomplete or unlocated medical records. The parameters studied were: age, sex, profession, consultation time, reason for consultation, history, functional, general and physical signs, data from paraclinical examinations, treatment received complications evolution. Data collection was done using a pre-established and tested counting sheet. Data entry was performed using Epi Data 3.1 software and analysis using Epi info 3.5.3 software. The interpretation was carried out by comparing the means obtained. The confidentiality of the identity of the patients was respected.
During the study, we had as difficulties, incomplete or not found files as well as badly filled files, without precision on the description of the clinical examination.
3. Results
3.1. Epidemiological Aspects
In 3 years, 55 patients were followed for diffuse cervico-facial cellulitis. The annual incidence was 18.33 cases per year. For a total of 1101 patients hospitalized in the department during the period, cases of diffuse CCF accounted for 4.99%.
Figure 1 shows the distribution of cases by age and sex.
The mean age was 41 years with a standard deviation of 15.1. The youngest patient was 15 years old and the oldest 85 years old. The predominance was male with a rate of 56.4% (31 men for 24 women) or a sex ratio of 1.29. Various professions have been listed. Table 1 shows the distribution of patients according to their occupation.
3.2. Diagnostics
The most common reason for consultation was painful cervico-facial swelling: 47 cases or 85.5% of cases. Dyspnea and skin necrosis were noted respectively by 5 and 3 patients, i.e. 9.10% and 5.4%.
The duration of evolution of the symptomatology was listed in the study. Table 2 shows the breakdown.
![]()
Figure 1. Distribution of patients by age and gender.
![]()
Table 1. Distribution of patients according to their occupation.
All the patients have started medication before going to hospital. Nonsteroidal anti-inflammatory drugs (NSAIDs) were used by 44 patients, i.e. 80% of cases, antibiotics were associated with NSAIDs by 26 patients, i.e. 47.3%. Traditional therapy was practiced by 8 patients, i.e. 14.5%. The latter brought together various practices, namely herbal teas, scarifications, poultices, herbal intraoral massages. Painful cervico-facial swelling was the most common reason for medical consultation (47 patients or 85.5% of cases).
The main risk factors identified were poor oral hygiene, auto-medication with non-steroidal anti-inflammatory drugs and diabetes. Different clinical signs were recorded. Table 3 highlights the characteristics of the different cases listed.
The injured teeth were molars. NSAIDs were taken by self-medication in the context of dental pain.
Table 4 summarizes the germs identified in the pus samples and their sensitivity to antibiotics.
Image 1 highlights the cervico-facial necrosis which allows the diagnosis of diffuse cervico-facial cellulitis to be made.
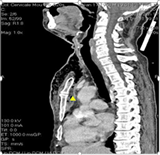
Image 2 includes CT sections of one of the patients, allowing the diagnosis of mediastinitis complicating a diffuse cervico-facial cellulitis.
![]()
Table 2. Distribution of patients according to consultation time.
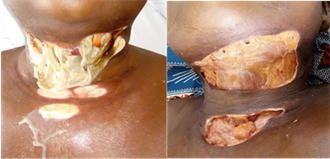
Image 1. Necrotic lesions in a case of a diffuse cervico-facial cellulitis.
 (a)
(a) 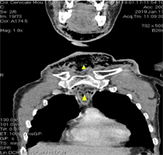 (b)
(b)
Image 2. (a) Retro-sternal collection on computed tomography, suggesting a medastinitis complicating a diffuse CCF. (b) Hyperdense image with supra clavicular and supra aortic gas traps reflecting mediastinitis.
![]()
Table 3. Clinical and paraclinical characteristics of CCFs.
![]()
Table 4. Distribution of germs according to sensitivity to antibiotics.
3.3. Therapeutic Aspects
The treatment was medical-surgical in all cases. The surgery consisted of an incision draining the pus (45 patients or 81.8%). It was performed under general anesthesia in 2 cases or 3.6%. Necrotic tissue was stripped and daily dressing was performed in all cases. Depending on the sensitivity of the germs in question, a bi or triple antibiotic therapy was administered parenterally then orally when the symptomatology improved. Antibiotic therapy was initially probabilistic. The combination of third-generation cephalosporin and demetronidazole was administered to 39 patients, i.e. 70.9% of cases. An aminoglycoside was added in 4 patients, i.e. 7.2% of cases.
Mechanotherapy was performed in cases of trismus. A stomatological consultation for dental care was carried out in cases of odontogenic cellulitis. The evolution was favorable for 47 patients or 85.5% of cases. Sequelae such as retractile scars and keloids were observed in 5 patients (9.1%). Two cases of orostoma (i.e. 3.6%) were recorded. Eight patients or 14.5% of cases died during hospitalization in septic shock in the tables.
4. Discussion
The incidence of CCFs is highly variable in the literature. This study found a mean of 18 cases per year. This figure is higher than that of Bouraima et al. in Parakou in northern Benin [7] who reported 14 cases per year. Lower frequencies had been noted in previous studies by Kpemissi in Togo in 1995 [8] and Lawson et al. [6] in Benin in 2012, with 8 cases per year and 7 cases per year respectively. Badou et al. [9] in Ivory Coast had noted a hospital prevalence of cellulitis of 3.5% with an annual incidence of 4.3 cases. These figures show that the prevalence of the disease is on the rise in the sub-Saharan region. The increase could also be linked to better care seeking by the community in recent years. Higher incidences have been reported by authors in northern countries. Indeed, Tran Ba Huy et al. in France [10] identified an average of 30 cases per year. Similarly in Morocco, Rouadi et al. collected 43 cases per year [11].
The cervical scar after healing can be unsightly. Image 3 is the photography of a patient after recovery
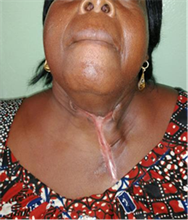
Image 3. Retractile cervicothoracic scar after diffuse CCF.
From this study, it appears that young adults were the most affected by CCF with an average age of 41 years (Figure 1). This finding is consistent with those of Ngouoni et al. [12] in Congo and Rakotoarison et al. [13] in Madagascar with average ages of 28 and 31 years respectively. Other authors have also reported a greater attack on individuals in their forties [4] [5] [14] [15].
Male subjects were the most affected in this study (Figure 1). The same was true in the work of Keita et al. [4] as well as Ngouoni et al. [12]. The male predominance found in the literature could be explained by the fact that women have a better immune response in various studies, including that of Rose et al. [16].
From this survey, the most vulnerable populations were those with low incomes (Table 1). The same remark was made by Konsem et al. in Ouagadougou [17] where 84.6% of patients exercised professions with a low socio-economic level (craftsmen, farmer). Other studies in the sub-region had found the same finding [9] [18]. It emerges from these various observations that the low-income socio-professional strata were the most affected.
Self-medication with NSAIDs was the main risk factor identified in this study (Table 3). The use of NSAIDs before the onset of CCF has been reported several times in previous works. This is the case of Miloundja et al. [3] in Gabon (87.5% NSAID use), EL Ayoubi et al. [19] in Morocco (48.3% NSAID use). The same findings were made by Salami et al. [20], in Treichville where 67.8% of patients self-medicated with NSAIDs, over an average duration of 5.6 days with extremes of 1 to 17 days. Hounkpatin et al. [21] in Parakou noted that the supply was done in pharmacies for 59.1% of patients. Indeed, NSAIDs have the advantage of calming the patient’s pain and fever, however, by inhibiting cyclo-oxygenase and they prevent the release of prostaglandins (mediators of inflammation) and thus reduce the patient’s immune defense [2]. They are the main factors backing the extension of CCF [3] [10] [22]. Due to their affordability and accessibility, they have been the first therapeutic resort for most patients.
Diabetes mellitus is a significant risk factor (Table 3). It concerned 15% of patients in this series. According to Hernandez et al. [23], diabetes mellitus was the first risk factor followed in decreasing order by alcoholism, smoking and chemotherapy. Badou et al. [9] noted HIV infection (9.6%) and diabetes (2.7%) as risk factors. HIV infection was also recorded in this study in 7.3% of cases. The resulting immunosuppression could justify the vulnerability of the terrain and the proliferation of germs responsible for cellulite. Aswan et al. [22] noted a hospital frequency of facial cellulitis added to diabetes of 9.8%; with 53% of patients discovering their diabetic state by chance. A systematic fasting glycaemia would therefore make it possible to identify and manage certain cases of diabetes in the face of cervico-facial cellulitis. Several authors [22] [23] agree that diabetes defines the induction and progression of cellulite; it produces leukocyte dysfunction, reduces phagocytosis chemotaxis. Additionally, hyperglycemia, a hallmark of diabetes mellitus, suppresses the immune system and enhances bacterial growth. A vicious circle is thus formed, infection-diabetes-infection. From our results, 5.5% of patients are pregnant. For Pucci Resi et al. [24], odontogenic infections have potentially harmful effects on pregnant women and therefore on fetal development. Of these odontogenic affections, it was counted: cervico-facial cellulitis in 30% of cases, Ludwig’s angina in 27.5%, abscess (submandibular and submental) in 23.2%, deep cervical suppuration complicated by mediastinitis in 17.8% and cerebral abscess in 1.5%. In fact, pregnancy is a period of relative maternal immunosuppression, making the body more susceptible to infections.
Human gingiva has been shown to be a target tissue for increases in estrogen and progesterone and estradiol-affected periodontal microvasculature that contribute to oral tissue alterations. Oral health is thus compromised during pregnancy due to all the hormonal factors and the significant alteration of the bacterial mass of the oral cavity, with a tendency towards a more anaerobic flora [25] [26]. The almost constant cervico-facial swelling was inflammatory and painful. On its own, it is evocative of cellulite. Several authors, notably Benzarti et al. [27], and Blancal et al. [28], have pointed out that painful cervico-facial swelling was the main reason for consultation in their series. The gangrenous CCF, characteristic by these snowy crepitations was objectified in approximately 34.5% of the cases. On the other hand, phlegmonous cellulitis was more represented in the work of Njifou et al. [29] in Cameroon (82%), Thiebaut et al. [30] in Dijon (82.3%). Forty percent (40%) of patients in this series had dyspnoea, sometimes accompanied by cough and chest pain. These signs of gravity made suspect: a diffuse CCF complicated by mediastinitis. Mediastinitis has been confirmed for some patients. In Morocco, Rouadi et al. [11] noted among the complications, 11% of mediastinitis and 1 case of thrombophlebitis of the internal jugular vein. These complications of mediastinitis, severe sepsis, and thrombophlebitis of the jugular vein were identified by Tran Ba Huy et al. in respectively 31%, 47% and 16% [10]. The high prevalence (91%) of dental origin was unanimously reported by authors such as Rakotoarison et al. [13] in Madagascar (74%) and Diallo et al. in Guinea Conakry (68%) [31].
Kouakou et al. [32] in Cote d’Ivoire mentioned in addition to dental caries (91.30%), skin infections (5.80%) and mandibular fractures (2.90%). The infected teeth causing more diffuse CCF were the molars in this study. This same remark was made by Sette Dias et al. [33] in Brazil and Obimakinde et al. [34] in Nigeria. Biologically, hyperleukocytosis with neutrophilic predominance was classic according to the authors [3] [27] [32] [34]. In the cultures of the series, a bacterial polymorphism with a predominance of aero-anaerobic saprophytic germs of the oral cavity was noted. The same germs were identified by Njifou et al. [29]. Panoramic dental radiography made it possible to objectify the causal tooth(s) (caries, radiolucent apical images, wisdom teeth included) and to rule out any mandibular causes as recommended by El Ayoubi et al. [19]. Medical treatment preceded and accompanied the surgical management of cervico-facial cellulitis. It revolved around resuscitation measures and antibiotic therapy. He also took into account the patient’s comorbidities. Antibiotic therapy was initially probabilistic and parenteral. For most authors, triple antibiotic therapy was appropriate: beta-lactam, imidazole and aminoglycosides [11] [13] [28]. With the antibiogram from the ECB result, other classes of antibiotics were used such as quinolones (Table 4).
Diffuse CCF requires flattening of all suppurated or necrotic areas. This implies a wide and extensible approach [19] [27]. In this study, surgical treatment was performed in 81.8% of cases. This result is similar to the conclusions of the series by Keita et al. [4] in Mali and by Rouadi et al. [11] in Morocco, which surgically took into account respectively 81% and 86% of cases. The drainage incision under anesthesia allows good resection of necrotic areas, facilitates washing, and thus promotes a rapid drying up of suppuration [8] [13]. General anesthesia was performed in 3.6% of cases in this study. In Madagascar, on the other hand, Rakotoarison et al. reports that 83.3% of patients benefited from a drainage incision under general anesthesia [13]. As soon as the general condition improved and the trismus regressed, patients with dental or periodontal lesions were referred for stomatological consultation. Bouraima et al. [7], as well as Lawson et al. [6] adopted the same attitude for odontogenic cellulitis. The case fatality rate of diffuse CCF was 14.5% in this study. Some authors have reported higher rates. Diallo et al. recorded 21% deaths [31], Keita et al. reported 28% deaths [18]. Despite the patient’s defects, these high scores are also explained by the delay in the consultation and the lack of means to access the prescribed treatment [4] [31].
5. Conclusion
The diffuse CCF is a serious condition in young adult males. The most common contributing factors were poor oral hygiene, use of NSAIDs, diabetes and HIV infection. It was essentially of dental origin. Complications such as septic shock and mediastinitis were frequent and very rapidly fatal. It is a medical-surgical emergency. Early and appropriate antibiotic therapy is necessary for the evolution of a cure.