1. Introduction
Enteromiuscallipterus [1] commonly called clipper or Congo barb belongs to the family Cyprinidae. Its native habitat is from Cote d’Ivoire through the Chad basin to Nigeria and Cameroon. E. callipterus is a tropical endemic fish that is of high economic importance in the region. It is noteworthy that the clipper barb is of great commercial interest in the aquarium industry [2]. It was originally described as Barbuscallipterus Boulenger in 1907. The clipper barb grows to a maximum length of 8 cm and a maximum weight of 0.18 kg (5.0 g). It has a slightly convex dorsal profile and short anterior barbels that do not extend beyond the eye. It has a light silverish-golden body but is darker on the back. The dorsal and caudal fins have a red-orange base while all other fins are colorless. It has a black spot on the first few rays of the dorsal fin but the tip of those rays are colorless [3]. E. callipterus has no distinct morphological feature that differentiates the male and female species except for the male being smaller than the female species.
Enteromius species are grouped as either small Enteromius or large Enteromius. The large Enetromius has many parallel stria; the dorsal fin has nine to eleven branched rays and a last hard ray with no denticles, and they are sometimes longer than 50 cm. The small Enteromius has scales with a small number of divergent stria; the dorsal fin has seven or eight branched rays, which are rarely longer than 10 cm. Hence, E. callipterus is a member of the small African Enteromius. In addition, chromosome studies have equally been used to unravel internal relationships among Enteromius fishes. Ploidy levels were found to be of more systematic value within the group compared to morphological characters [4]. For example, [5] shows that the number and size of barbels on the fish can vary depending on the size of the individual or on particular populations of the same species. Three ploidy levels are recognized and are: diploid (about 50 chromosomes), tetraploid (about 100 chromosomes), and hexaploid (about 150 chromosomes) [6] - [11]. The karyological findings support the dichotomy of Enteromius into small and large groups as the chromosome numbers reported for the small ones are generally within 48 and 50 while the chromosome number for the large ones are within 100 and 150. Dimorphism in the chromosomes of the different sexes of Enteromius fishes has also been studied [12]. However, there is no data on the karyotype of E. callipterus. Since it is known that karyotyping and chromosomal analysis gives a more detailed explanation for the diversity and changes between species, this study assesses the karyotype of the male and female species of E. callipterus.
2. Materials and Methods
2.1. Fish Collection
Live samples of Enteromiuscallipterus were collected from the Tahoss River, Plateau state, using a frame net, during a fishing expedition in January 2019. Identification of the fish was done using identification keys by [3] and [13].
2.2. Chromosome Extraction Procedure
The chromosome extraction procedure was initiated by injecting the fish with the appropriate dosage of colchicine from a stock of 0.05% wt./vol colchicine. The injection was made at the muscle mass at the base of the dorsal fin at a dosage of 0.02 ml per gram wet weight. The specimens were sacrificed three hours after the colchicine treatment and the gills were removed. The gills from each specimen were treated separately. The gills excised were placed in a hypotonic solution of 0.56% KCL for 30 minutes. During the first 10 minutes, the tissue was squashed to homogeneity within a mortar using a pestle. The cell suspension was decanted into a 9 ml centrifuge tube and spun at 1000 rpm (revolution per minute) for 10 minutes in a 90-1A Model Life care centrifuge. The supernatant was carefully removed with the aid of a 2.5 ml glass pipette leaving about 1 ml of the solution containing the cell pellets.
2.3. Fixation of Cell Pellets
The pellets were suspended in freshly prepared modified Carnoy’s fixative (3 methanol: 1 acetic acid), and then centrifuged at 1000 rmp for 5 minutes, and the supernatant decanted. The fixation procedure was repeated twice to ensure proper fixative of the cells prior to spreading of the cells. A final cell suspension was prepared by adding a few drops of fresh Carnoy’s fixative to the pellet.
2.4. Chromosome Spreading
A portion of the cell suspension was drawn with a 2.5 ml glass pipette and two to three drops of it were dropped on a clean, cold and wet glass slide from a height of about 60 cm, with the slide at an angle of 45’ between the thumb and the index finger. The glass slides were previously kept in the refrigerator set at 10˚C. Four slides each were prepared for both species. Each slide was labelled and left to dry on a Photax dish warmer 2a Model slide warmer for about 24 hours at a temperature of 60˚C.
2.5. Staining of Slides
The cells were stained with 6% stock Giemsa stain which had been prepared earlier. The dried slide were dipped into the staining rack containing the Giemsa stain and allowed to stain for about 25 minutes. The stained slides were rinsed with distilled water and dried on a Photax dish warmer 2a Model slide warmer set at 60˚C.
2.6. Photomicrography of Spread
After about 12 - 24 hours of drying on the slide warmer, the slides were viewed under the Omax G013055005 Model trinocular light microscope. The chromosomes spreads adjudged well under the lower magnification were further examined at the x100 objective. Photomicrography of the spreads was also taken at x100 objectives using A3514OU Model camera attached to the microscope. The morphology, diploid number (2n) and the fundamental number of autosomal arms (NFa) of the chromosome arms were determined. The chromosomes were karyotyped manually from printed copies of the photomicrographs of the mitotic metaphase chromosome spreads. The chromosomes were cut-out and homologous chromosomes were paired based on their length, morphology and position of centromere. The length of each chromosome and idiogram were determined using Karyotype software. The measurements and nomenclature were according to [14].
3. Results
The chromosome spread obtained for E. callipterus male is shown in Plate 1 while Plate 2 shows the karyotype of E. callipterus male. The diploid chromosome number is 50 while the fundamental number of autosomal arms (NFa) is 82 and karyotype formula is 2n = 14M + 4m + 32t. The chromosome nomenclature shows that E. callipterus male chromosomes 1-5 are metacentric chromosomes with each one having the centromere in the median region (m). However, chromosomes 6 to 25 are telocentric chromosomes. The morphology of the chromosomes of the male sex in form of an idiogram is presented in Figure 1. On the other hand, Table 1 shows the chromosome measurements and nomenclature of E. callipterus male.
For the female, none of the spreads obtained were made up of chromosomes number either equal or below 2n = 50. The best of the spreads are presented in Plate 3 to Plate 4. Plate 3 shows the first spread with its karyotype in Plate 4 while Plate 5 shows the second spread with its karyotype in Plate 6. The diploid chromosome number for the first spread is 54 while that of the second spread is 58.
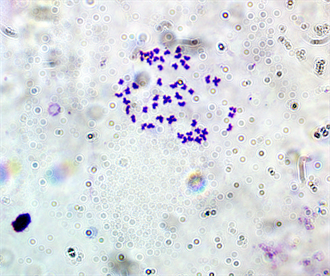
Plate 1. Mitotic metaphase chromosome spread of E. callipterus male(2n = 50).
![]()
Figure 1. The idiogram of the karyotype of E. callipterus male.
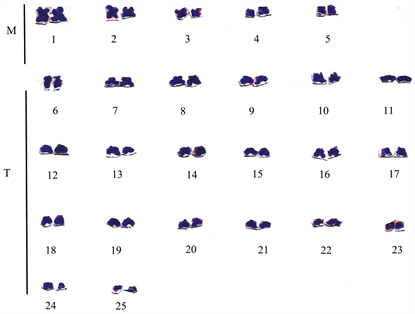
Plate 2. Karyotype of E. callipterus male.
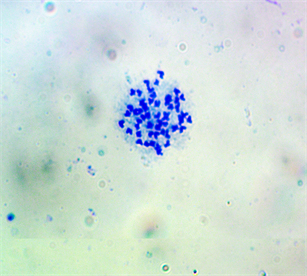
Plate 3. First mitotic metaphase chromosome spread of E. callipterus female (2n = 54).
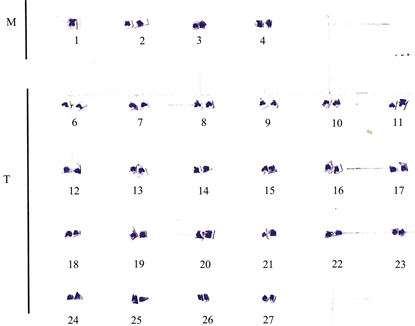
Plate 4. Karyotype of the first spread for E. callipterus female. M: metacentric; T: telocentric.
![]()
Table 1. The chromosome measurements and nomenclature of E. callipterus male.
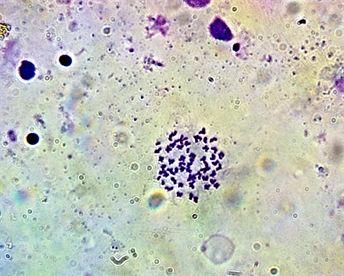
Plate 5. Second mitotic metaphase chromosome spread of Enteromius callipterus female (2n = 58).
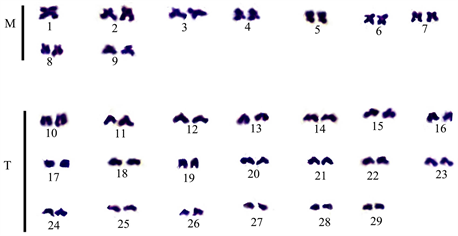
Plate 6. Karyotype of the second mitotic spread for Enteromiuscallipterus female. M: metacentric; T: telocentric.
4. Discussion
The diploid chromosome number of 50 exhibited by the male sex of the E. callipterus is consistent with the reported chromosome number of the species in the genus Enteromius. [15] reported that out of the 25 African small Enteromius species that have been karyotyped and reported, 18 are of the chromosome number of 50, while the other 7 are of the diploid chromosome number of 48. In general, the Cypriniformcytotaxonomy reveals variation in the diploid chromosome number ranging from 42 in Acheilognathusgracilis to 446 in Diptychusdipogon [16] [17]. However, 2n = 50 is the most frequent chromosome number, which represents a basal pattern for the whole group.
Karyotyped diploid cyprinids are generalized by relatively small chromosomes, which makes locating the chromosomes and identifying their orientation very difficult. The chromosomes are characterized by a centromere position placed gradually from a terminal point to a median point [10] [11] [18]. Comparatively, the spread obtained for the E. callipterus female was smaller making identification of the orientation of the chromosomes even more challenging. Another peculiarity in the female E. callipterus chromosome is the number. Contrary to the typical 48 - 50 diploid chromosome number, the E. callipterus female spread presents the diploid chromosome number of 54.
Sexual dimorphism at the chromosome level has been characterized among organisms [19]. However, sexual dimorphism at the chromosomal level in the cyprinid is scarce. The sex chromosomes are believed to have mostly remained undifferentiated across many taxa. The results obtained for the female E. callipterus strongly suggest that the species exhibit theXX and XO sex chromosome dimorphism pattern. This resulting study is presented as preliminary investigative research on sexual dimorphism of E. callipterus and further studies using the molecular cytogenetic method are recommended to confirm this observation. The study, therefore, concluded that the male E. callipterus has the diploid chromosome number of 50 while the diploid chromosome number for the female E. callipterus could be between 54 and 58. The study also suggests sexual dimorphism at the chromosome level in the species.