Results of the Management of Gastrointestinal Stromal Tumors (GIST): About 6 Cases at the Vichy Hospital Center (France) ()
1. Introduction
Gastrointestinal stromal tumors (GIST) are the most frequent digestive sarcomas and represent 18% to 20% of all soft tissues’ sarcomas [1] [2] [3]. These are mesenchymal tumors of the gastrointestinal tract with a high risk of malignant transformation. Their diagnosis is based on histology and demonstration of C KIT expression by immunohistochemistry or a KIT or PDGFRA mutation, by molecular biology [1] [2]. They can develop on all segments of the digestive tract from the esophagus to the anus. They are often asymptomatic. The diagnosis is fortuitous in the majority of cases. The curative treatment remains surgical resection, combined with a selective tyrosine kinase receptor inhibitor (Imatinib) as an adjuvant or as a neo-adjuvant to improve the morbidity and mortality associated with GIST [2] [4]. The objective of our study was to analyze the results of the surgical management of gastrointestinal stromal tumors (GIST) at the Vichy Hospital Center.
2. Materials and Methods
This was a 10-year retrospective study from January 2010 to December 2020 in the surgery department of the Vichy Hospital Center. Six patients were collected during this period. Patient data was collected using ORBIS software (patient medical record management software). The inclusion criteria were all patients received during this period for gastrointestinal stromal tumors confirmed by histology, immunohistochemistry. Patients whose diagnosis was not confirmed by histology were excluded. The parameters studied were epidemiological, diagnostic, therapeutic, anatomopathological and prognostic data.
3. Results
There were 5 men and one woman with an average age of 72.16 years [58 - 80 years]. The mean evolution time was 8 months (0 - 14 months). The diagnosis was fortuitous in 2 cases. Atypical abdominal pain was found in 1 case and chronic anemia in 2 cases. One case was received in an array of peritoneal irritation syndrome. Arterial hypertension was found in 5 patients, diabetes in 2 patients and a history of Fundoplication. Esogastroduodenal fibroscopy was performed on 5 patients. Echo-endoscopy with biopsy and histological examination of the biopsy specimen made it possible to make the diagnosis in 5 cases. The fusiform type was the predominant histological form in 4 cases and 2 cases of epithelioid form. The stomach was the most common location, especially at the level of the fundus (Image 1). Neoadjuvant chemotherapy was performed on one patient. Surgery was curative and laparoscopic in 4 patients. Atypical gastrectomy of 2 cm on either side of the tumor was possible in 4 cases. A total gastrectomy with splenectomy was performed after a good response to Imatinib targeted therapy at a dose of 400 mg/day for a period of 9 months in one case (Image 2). The resection margins were healthy in 5 cases. The mitotic index was low, less than 5 mitoses out of 20 fields in 3 cases with a Ki 67 of less than 1%. Two had respectively a mitotic index of 13/20 and 53/20 and a Ki 67 which were around 60% and 70%. Molecular biology was performed on 2 patients and showed a C.1676T>A p. (Val559Asp) and the C.1669_1674 mutation of ITGGAAG and protein alteration P (Trp557 Lys558del) on exon 11 of the C-Kit gene. An absence of mutation was noted on exon 9 and 18. Table 1 summarizes the results of the anatomopathological examination, molecular biology and TNM classification of the tumours. At the end of the radiological and histological assessment, the diagnosis of localized GIST was retained in 4 cases and metastatic in 2 cases. Adjuvant chemotherapy based on Imatinib at a rate of 400 mg/day was initiated in 3 patients for a period of 6 months. Disorders of the digestive tract such as diarrhoea, vomiting and skin lesions (cutaneous rash) were the side effects linked to the targeted therapy. One patient presented with a fistula of the oeso-jejunal anastomosis on D6 postoperatively controlled by drainage and antibiotic therapy. After an 18-month follow-up, there is complete remission of the lesions. There was no local or metastatic recurrence. Mortality was zero. During the surgery, all of the patients were followed up with surgeon, oncologist.
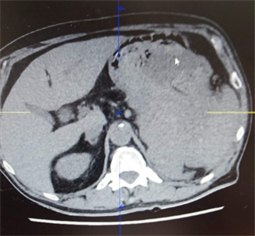
Image 1. A big fundic GIST invaded the spleen, before the treatment with Imatinib.
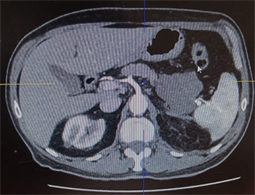
Image 2. Tumor regression after 9 months of treatment with Imatinib 400 mg/j.
![]()
Table 1. Authors. contributions: they contributed to the production of the final document.
4. Discussion
GIST correspond to a proliferation of interstitial cells of Cajal (or their progenitors), most often fusiform, sometimes epithelioid, rarely pleiomorphic arising from the muscularis of the digestive tract preferentially from the stomach or the small intestine. They are rare tumors and represent about 1% to 2% of malignant gastrointestinal tumors [3] [5] [6] [7]. It is the most common mesenchymal tumor of the gastrointestinal tract.
They occur more frequently in adulthood, with an average age of 60 years and an equivalent frequency in both sexes [2]. In our series, the age was 72.12 years, higher than those found in the literature.
GISTs are preferentially located in the stomach (60%), small intestine (25%), colon and rectum 6%, esophagus 0.7% and other locations 5.5% [2] [3] [8]. As in the literature, gastric localization was the most frequent in our series.
There are no risk factors apart from a few rare tumor syndromes such as neurofibromatosis type I, Carney-Stratakis syndrome in which germline mutations of SDHB (succinate dehydrogenase complex, subunit B) have been detected, and the triad of Carney [9]. Concerning the clinical presentation, the GIST are symptomatic in 70% of the cases, 20% of the asymptomatic cases and of fortuitous discovery and in 10% of the cases of post mortem discovery. The signs are not specific and depend on the location of the tumour. The main signs are represented by bleeding (30% to 40%) and atypical abdominal pain (60% to 70%) [1] [8] [10].
In our series, atypical symptoms such as chronic anemia, poorly systematized abdominal pain led to the diagnosis after the realization of echo-endoscopy with biopsy. These tumors remain asymptomatic for a long time, until they become large or cause complications making their discovery fortuitous during an upper digestive endoscopy in about 20% of cases [10] [11]. In our study, the systematic performance of CT scans for any patient seen in the emergency room in the context of fever or dyspnea during the COVID pandemic resulted in the incidental discovery of GIST. Digestive endoscopy and ultrasound endoscopy with biopsy play an important role in the diagnosis and management of these tumours. Superficial (mucosal) endoscopic biopsies are generally negative, as the tumor develops in the muscularis of the digestive tract [8]. The biopsy can be performed by echo-endoscopic, percutaneous or surgical approach, and must be discussed on a case-by-case basis during a multidisciplinary meeting [12]. Such a procedure involves a risk of hemorrhage, and potentially of peritoneal dissemination when performed percutaneously or laparoscopically. It is generally recommended preoperatively in sarcomas. However, in GIST, it is especially essential in the event of diagnostic doubt, doubtful resectability, major or mutilating surgery, or the need for initial medical treatment. Biopsy is nevertheless essential if the choice of treatment requires a certain histological diagnosis. In case of doubt diagnosis with a tumor requiring first chemotherapy or a different surgical procedure such as lymphoma or a locally advanced tumor, the biopsy retains its interest [12] [13].
The genesis of GIST involves two kinds of receptors: C kit and PDGFR. Mutations in these receptors are heterozygous gain-of-function type. The mutations are in 85% of the cases on the gene encoding the protein C kit and preferentially seat in the juxta membrane on exon 11, can also concern the extra-membrane zone of the protein on exon 9. The mutation on the gene PDGRF is less common and accounts for 10% - 15% of cases [14]. In our series, the mutation on exon 11 of the C-Kit gene was the most found. Immunohistochemistry found antibodies, the most common of which are anti-CD34, anti-CD117 and anti-DOG1 antibodies. These results are consistent with those found in the literature [14] [15]. Mutations are also predictive of therapeutic response by C kit inhibitors. Thus, when a duplication of exon 9 of the C-Kit is encountered, the dosage of Imatinib in metastatic GISTs will be doubled and GISTs with a partial mutation (PD842V of PDGFRA) are resistant to this treatment [2] [16].
Any diagnosed GIST must be resected because it is potentially malignant [7] [13]. Therapeutically, endoscopic surveillance is licit for GIST less than 2 cm localized in the stomach. On the other hand, GIST of the hail or the rectum, whatever the size, a resection is the rule because of the progressive risk, especially if the mitotic index is high [7] [17]. Sixty percent of patients have GISTs localized and the treatment is based on surgery. The imperatives of this surgery are based on complete excision R0, with resection margins of 1 to 2 cm, without rupture of the tumor capsule [7] [17]. Laparoscopic resection of GIST, by experienced surgeons, is feasible and comparable to laparotomy in terms of quality of excision, postoperative complications and risk of recurrence. It has the advantage of resulting in a shorter hospital stay. The size of the lesion no longer seems to be a contraindication to this approach. Therefore, laparoscopic surgery for gastric GIST seems to improve postoperative outcomes and does not worsen the oncological prognosis [18]. However, when the tumor is large, it is preferable to perform a laparotomy to avoid tumor invasion, which definitely worsens the prognosis. In our series, 4 patients underwent a laparoscopic approach. Lymph node dissection is not systematic, because lymph node metastases are rare and the risk of lymph node recurrence is limited, except in pediatric forms. In case of doubt, lymph node picking is recommended [7] [15] [19].
It is indeed essential to avoid intraoperative invasion of the tumor, which affects overall survival. Laparoscopic resection is possible, but should be avoided in the case of a large tumor with significant cystic, hemorrhagic and/or necrotic changes [18]. Recently, several reviews have studied the management of small gastric GIST localized at the esophagogastric junction or the pylorus by endoscopic laparoscopic resection. This technique is safe, reliable and preserves gastric functions [7].
The management of GISTs has been completely modified by the introduction of Imatinib, which has transformed the evolution of metastatic forms and recurrences [20]. Preoperative chemotherapy with Imatinib is indicated in rectal GIST to avoid an attempt at abdomino-perineal amputation, GIST of the esophagus or duodenum and large GIST of the stomach which requires total gastrectomy [5] [20]. A patient in our series, carrying a gastric GIST with splenic metastasis, after treatment with Imatinib 400 mg/day for an average duration of 9 months made the tumor resectable with a regression of the tumor size which went from 15 to 7 cm. New therapies with ipilimumab, nivolumab and echo-endoscopic resection are in progress and the results are promising [5] [14].
5. Conclusion
GISTs are the most frequent sarcomas of the gastrointestinal tract characterized by an overexpression of C Kit in immunohistochemistry and by activating mutations of receptor tyrosine kinases. Surgical excision is the rule for localized forms. Treatment with Imatinib has revolutionized the management of metastatic and recurrent forms, but also to avoid mutilating surgery on certain organs such as the rectum and the esophagus. The prognosis is correlated to the location, the size of the tumor and the mitotic index. The laparoscopic approach retains its interest in the management of these tumors.