Competitive Adsorption of Metal Ions on Peanut Testa (Arachis hypogaea L.) Extract Using Cation Exchange Resins ()

1. Introduction
Industry wastewater and effluents are commonly contaminated with a variety of heavy metal ions rather than a single one. Heavy metals, like all poisonous substances are known to interfere with biological activities. Reference [1].isted taunted growth, reproductive impairment, cancer and mortality among the effects of heavy metal pollution in man. Additionally, organ damage and endangered life were equally reported [2]. Reference [3].xplained that surplus Fe will deposit in the body organs and synthesize non-transferring-bound Fe. Lethargic protein metabolism and associated sterility in mammalian males are signs of the toxicity of Zn [4]. While partial blindness and imperfect hormonal activities are minor symptoms of Pb poisoning, undeveloped grey matter and consequential low acumen are indications of Pb build-up in humans [5]. Reference [6].eported carcinogenicity of Ni compounds to animals when in excess. This triggers hypoglycemia, nausea, headache and respiratory diseases [7]. Excess Cu manifests as diarrhea and dehydration, anaemia and paralysis amid other illnesses in vertebrates and distress benthos through organ damage and ultimate cessation of the animal’s life [8].9].
Other than reported oxidative stress in affected plants, [10].numerated sedentary nutrients uptake and stumpy growth, chlorosis, low fruit yield, metabolic syndromes and nitrogen fixing disability in legumes as signs of heavy metals contamination in plants. The UNEP Joint Report (2010) [11].inked the reported annual 1.8 million infantile deaths from waterborne diseases to heavy metal polluted industry wastewater and effluents discharged into the world’s waterways [12]. Corroborating, H. R. H. Prince Willem-Alexander of The Netherlands connected poor human health, declining agricultural yield and insignificant socio-economic growth to indiscriminate discharge of wastewater into water courses and underscored its militating role against the advocacy for hygiene practice and ecosystem sustainability [13].
The above narrative shows the need to identify pollutant heavy metal ions in wastewater and remove them before releasing into the environment to avoid their build-up beyond the WHO permissible limits for industry discharges. The initiatives and fiscal commitments to good health have awakened the consciousness and stimulated interest and effort towards the purification of heavy metal polluted water. Moreover, the advocacy in developed countries for reduced presence of metal ions in water has generated support for the development of feasible ways to remedy heavy metal contaminated water and industry effluents and prompted stringent guidelines for their discharge into the environment [14].15]. Citing the activism of global institutions to the objectives, [14].16].ighlighted the synergy between contemporary researchers on the development of safe, economical as well as practical and sustainable technology to decontaminate heavy metal polluted water.
Literature is awash with publications about methods to eliminate single metal ion pollutants in water but has comparatively devoted little consideration to explore techniques that purify multi-elements contaminated industry wastewater and effluents. Reference [17].mphasized the importance of wholesome treatment that removes heavy metal ions (typified by multilevel contamination) in aqueous systems.
In Nigeria and other countries characterized by large scale peanut cultivation, large volume, (nearly 0.74 million metric tonnes) of polyphenol-rich, peanut skin or testa is generated as waste from peanut processing industries [18].19]. This polyphenol-rich peanut testa is rich in catechin [20].21]. Figure 1 presents the structure of catechin having five hydroxyl groups which are rich in lone pairs.
Despite under-utilization of peanut testa as supplements in animal feeds [22]. much of it is littered resulting in a myriad of environmental pollution problems [23].24]. Consequently, innovative ways to harness this waste for gainful exploitation through creation of value-added products are desirable. Conversion into cation exchange resins for eliminating toxic metal ions in industry wastewater would ensure utilization and validate the tripartite goal of waste minimization, environmental clean-up and wealth creation from peanut testa that has abetted the breeding of nauseating pests. The aim of this study is to investigate the preferential adsorption of Fe2+, Ni2+, Pb2+, Cu2+ and Zn2+ in multi-elements contaminated water using environmentally friendly adsorbents derived from peanut testa extract polyphenols.
2. Materials and Method
All the chemicals used in this study were of analytical grade, supplied by Idex Scientific Supplies Company, Ltd, Aba, Nigeria and were used without further purification. The sample collection, processing as well as storage of peanut testa (PT) and the extraction of polyphenols from the PT have been reported [25].
2.1. Preparation of Metal Ion Solutions
Primary stock solutions containing 100 ppm of the following metal ions: Zn2+, Pb2+, Fe2+, Cu2+ and Ni2+ were prepared by dissolving 0.1100, 0.0400, 0.1245, 0. 0785 and 0.1683 g of ZnSO4∙7H2O, Pb(NO3)2, FeSO4∙7H2O, Cu(CH3COO)2∙H2O and (NH4)2SO4∙NiSO4∙6H2O respectively in a little quantity of DI H2O in separate 250 ml volumetric flasks and topping to volume with DI H2O. This gave the individual elements primary stock solutions.
Subsequently, a multi-elements primary stock solution containing 100 ppm of each of the same metal ions was prepared by dissolving the same amount of the corresponding metal salts in a little quantity of DI H2O in separate 50 mL beakers and transferring all, together with the rinses to a 250 mL volumetric flask before diluting to volume with DI H2O.
Thereafter, 10 ppm and 5 ppm working solutions of the individual element solutions and a multi-elements solution were prepared. Five (5) mL of 50% HNO3 was added to all the preparations before diluting to volume to prevent precipitation of the metals as salts.
2.2. Preparation of PTE Resins
PTE was reacted with POCl3 and CSA through different synthetic procedures to obtain crosslinked, substituted, phospho-sulphonated and sulphonated PTE resins.
2.2.1. Preparation of Crosslinked-PPTE
PTE was crosslinked with POCl3 by following the reported procedure [26].s modified [13]. Having stirred PTE (25 g) in pyridine (250 mL) held in a 1 L three-neck flat bottom flask for 12 hrs at RT in a fume hood, the flask was equipped with a thermometer, dropping funnel and a reflux condenser. Thereafter, a freshly prepared mixture consisting of POCl3 (5 mL) and 25 mL of dichloromethane (DCM) was added by drops through the funnel over 30 mins. When the set up was clear of fumes, the flask was transferred to a paraffin oil bath and heated at reflux for 3 hrs at 90˚C with continued stirring. At the end of the process, the oil bath was brought down, the flask detached and set to cool down. The content was poured into a 600 mL beaker containing 200 mL of ice H2O, stirred thoroughly with a glass rod and rapidly filtered on a white band filter paper. The residue was copiously washed, first with DI H2O until it was free from the smell of pyridine. Again, 100 mL of 0.1 M HCl and DI H2O was used until the filtrate tested neutral to methyl red indicator. In the last part, it was washed with 100 mL of methanol (MeOH) and dried overnight in an oven at 65˚C to yield 65.43% POCl3-crosslinked PTE (Crosslinked-PPTE) resin.
2.2.2. Preparation of Substituted-PPTE
PTE polyphenols was converted into a cation exchange resin with phosphate (
) end groups through a phosphorylation reaction using a PTE: POCl3 mixture of a different proportion. Substituted-PPTE was synthesized by the procedure for Crosslinked-PPTE but by adjusting the amount of POCl3 used in the reaction. PTE (25 g), previously stirred for 12 hrs in pyridine (250 mL) was reacted with a freshly prepared mixture consisting of POCl3 (15 mL) in DCM (90 mL). The POCl3-DCM mixture was added by drops over 1½ hrs and the rest of the procedure for the preparation of Crosslinked-PPTE followed to obtain 71.05% of POCl3-substituted PTE (Substituted-PPTE) resin.
2.2.3. Sulphonation of Crosslinked-PPTE Using CSA (PS-PTE)
Functionalization of Crosslinked-PPTE using CSA was achieved through an adjustment of the method described and reported [13].27]. Crosslinked-PPTE (25 g) was stirred to homogeneity in pyridine (250 mL) at RT according to the method for preparing Crosslinked-PPTE and Substituted-PPTE. After adding a freshly prepared mixture of CSA (5 mL) in DCM (40 mL) by drops, the rest of the procedure was followed to obtain 68.54% of POCl3-crosslinked and sulphonated-PTE (PS-PTE) resin.
2.2.4. Sulphonation of PTE (SU-PTE)
The phenolic groups in PTE polyphenols were adapted to
groups by treating with CSA according to the procedure for the phosphorylation of same but by reacting PTE (25 g) stirred in pyridine (250 mL) with CSA (8 mL) in DCM (40 mL) mixture. The yield was 76.87% of sulphonated-PTE (SU-PTE) resin.
2.3. Resins Characterization
The techniques used for the characterization of the synthesized resins, the starting materials and Bio-Rex 70 together with the instrument used, the models and operational ranges have been reported [25].
2.4. Ion Exchange and Adsorption Studies
Prior to use for the individual batch adsorption experiments, PT, PTE, Crosslinked-PPTE, Substituted-PPTE and PS-PTE as well as SU-PTE and Bio-Rex 70 were converted to the hydrogen ion (H+) forms (activation) by filtering and washing to neutrality, an overnight dilute HNO3 saturated portion and oven drying for 4 hrs at 60˚C. Then, each HNO3 activated adsorbent (1 g) was transferred into separate 50 mL solution containing 10 ppm of Zn2+, Pb2+, Fe2+, Cu2+, and Ni2+ in a plastic bottle. 5 g of each resin and Bio-Rex 70 was also transferred into separate 50 mL of the multi-elements solution containing 10 ppm of the metal ions in five different batch set-ups. Finally, 2.5 and 50 g of PS-PTE resin was transferred into separate 100 mL multi-elements solutions containing 5 and 100 ppm respectively of the metal ions in two other set-ups. The pH of the solutions which were administered using different delivery pipettes was determined and adjusted to 6 ± 0.5 with 0.1 M HCl or 0.1 M NaOH before the introduction of the resins. The caps were immediately secured, the bottles positioned on a Nuve SL 250 horizontal bench shaker and agitated at 150 rpm (revolutions per minute) for 2 hrs at RT. Thereafter, the bottles were unfastened and the suspensions rapidly filtered on separate Whatman No. 44 folded filter paper. The concentration of each metal ion in the filtrates was determined using Flame Atomic Absorption Spectrophotometer (AAS) and the amount of the metal ion (ppm) removed from the solutions by individual adsorbents at equilibrium Qe, computed from the model given in Equation (1):
(1)
where: Co and Ce are the initial concentration and concentration (ppm) of the metal ion in the solution at equilibrium while V and W respectively are the volume of the solution and the weight of the adsorbent (g) used in the experiment.
3. Results and Discussion
3.1. Modification with POCl3
The modification of PTE with the electrophilic reagent POCl3 as reported [25]. took place via the polycondensation of PTE polyphenol units (demonstrated using catechin molecule) by the interaction between the hydroxyl hydrogen (H) of the catechin and Cl− of POCl3. The reaction progressed by two distinct routes, to yield Crosslinked-PPTE and Substituted-PPTE. In order to obtain the former, POCl3 reacted at a single -OH site of adjacent catechins, condensed them into a bridge with the elimination of HCl molecules. This resulted to the formation of a -P-O- bond of a phosphate ester (
) on either sides of the newly created -P=O bond on catechins and a replaceable -OH group (a PTE phospho-mono-anion). Substituted-PPTE, on the other hand, formed when POCl3 reacted with an OH group of a unit PTE catechin, substituting the phenolic hydrogen (H) and replacing it with a terminal
group with two ionisable -OH groups (PTE phosphor-di-anion). The reactions for the formation of the Crosslinked-PPTE (a) and Substituted-PPTE (b) are illustrated in Scheme 1.
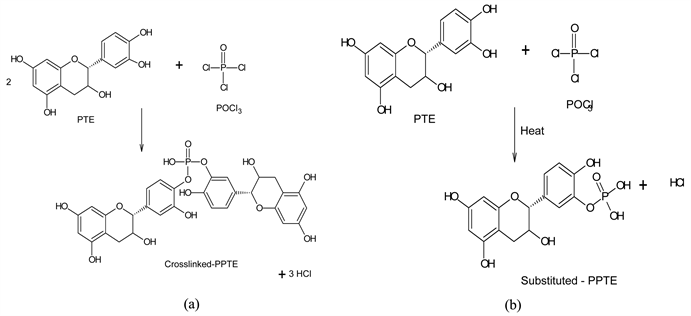
Scheme 1. Formation of Crosslinked-PPTE (a) and Substituted-PPTE (b).
3.2. Sulphonation of PTE and Crosslinked-PPTE with CSA (SU-PTE and PS-PTE)
Functionalization of PTE and Crosslinked-PPTE was accomplished by direct sulphonation using CSA. In the case of Crosslinked-PPTE, the process boosted the electron density around the -P=O bond through an electrophilic substitution that replaced the hydroxyl hydrogens (H) in PTE-catechins and Crosslinked-PPTE with negatively charged
groups. The reaction paths leading to the conversion of PTE and Crosslinked-PPTE to SU-PTE and PS-PTE respectively are shown in Scheme 2 and had been reported [25].
3.3. FTIR Spectra of PT and PTE
Table 1 outlines the characteristic absorption bands found in the FTIR spectra of PT and PTE [28]. The broad bands at 3431/3423 cm−1 and the medium peaks at 1377/617 cm−1 respectively are ascribed to stretching and in- and -out-of-plane bending movements of -OH groups of the polyphenol (which are 5 in catechin) units of these starting materials. The 2 IR bands found at 2928/2926 cm−1 and the lone band at 1142 cm−1 are due to stretching and bending of the sp3 -C-H bond of the cyclic ether middle ring of testa catechins. The two IR absorption bands at 1629 and 1528 cm−1 are credited to -C=C- bonds in the adjacent rings of catechin and whereas the -C-O-C- stretch of the cyclic ether central ring absorbed IR radiation at 1160/1034 cm−1, absorption bands due to its -C-H stretch showed at 823/668 cm−1.
3.4. FTIR Spectra of Modified PTE Resins
In addition to the absorption bands in the 3400, 2900 and 1600 cm−1 regions corresponding to the -OH, -CH2 and -CH groups of the starting material which also showed in the spectra of all the products, the synthesized resins displayed additional IR absorption bands [28].etween 1400 and 900 cm−1 depending on the reagent with which the chemical modification was performed.
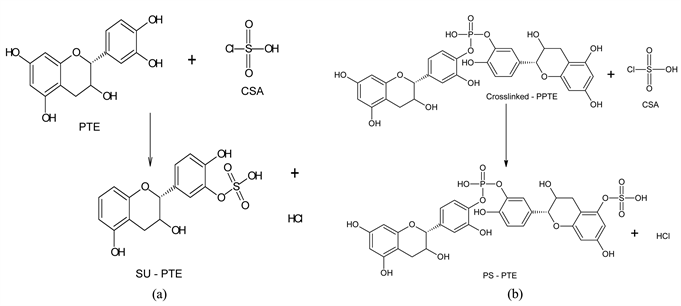
Scheme 2. Formation of SU-PTE (a) and PS-PTE (b).
![]()
Table 1. Characteristics bands in the FTIR spectra of PT and PTE.
From the results obtained the intensities of the -OH stretches at 3431/3423 and 1377/617 cm−1 (Table 1) were higher in all the starting materials when compared to the products formed (Table 2 and Table 3). The intensities of the absorption bands due to stretching and in-plane and out-of-plane bending movements of the different -OH groups in the spectra of Substituted-PPTE; Crosslinked-PPTE; PS-PTE; SU-PTE and PTE were 3.5 and 3.9; 3.4 and 3.7; 3.4 and 3.8; 3.3 and 3.6 and 1.8 and 2.4 respectively.
A polymer is a high molecular weight substance resulting from a systematic arrangement of numerous repeat units within a chain structure while a monomer is the basic unit or group of units (in case of a co-polymer) that is repeated in the chain. In the present study, PTE catechin units are the monomers while the two crosslinked products: Crosslinked-PPTE and PS-PTE represent the polymers. The observed increased intensities of the -OH absorption bands in these products is a consequence of the bridge created during the crosslinking of PTE catechin units to produce the Crosslinked-PPTE polymer. Moreover, the
![]()
Table 2. Characteristics bands in the FTIR spectra of Crosslinked-PPTE and substituted-PPTE.
![]()
Table 3. Characteristics bands in the FTIR Spectra of PS-PTE and SU-PTE.
collective intensity of the -OH groups in a Crosslinked-PPTE polymer chain (PTE-O-P-O-PTE)n offsets the intensity of the 5 -OH groups of individual PTE catechin units. In addition, the contributions by the -OH groups of the phosphonic, sulphenic and sulphonic acids as well as those of the phosphates, sulphone and sulphates may have further boosted the strength of the -OH absorption bands.
While the IR bands in the 1200 and 960 cm−1 region are assignable [28].o the -C-O-P- stretch of phosphate esters in Crosslinked and PS-PTE, these bands are not present in the spectra of Substituted-PPTE. The intensity of the -C-O-P- absorption band showed to be higher in the spectra of Crosslinked-PPTE relative to that of Substituted-PPTE. This is due to the effect of the twin -C-O-P- bonds contributed by the R-O-P-O-R bond of individual catechin units in Crosslinked-PPTE against the lone -C-O-P- bond in Substituted-PPTE.
IR absorption bands ascribed to the asymmetric stretch of the -S=O bond of the
and
groups were seen at 1450/1345 cm−1 while the same for the asymmetric and symmetric stretch correspondingly of the O=S- bond in O=S=O showed at 1323/1300 and 1160/1125 cm−1 in the spectra of PS-PTE and SU-PTE.
While PS-PTE produced absorption bands conforming to IR movements at eight locations, including 2700/2550; 1260/230; 1200/1100 and 1050/970 as well as 1410; 1450/1345; 1323 - 1300; 1160 - 1125 and 1062 - 1034 cm−1 respectively confirming the presence of all H-O-P=O; -C-O-P=O-; -P=O; -P-O-C-; C-O-S; O=S=O;
,
and -S-O-H functional groups in the same molecule, IR absorption bands present at 1420/1100; 1450/1345; 1323 - 1300; 1160 - 1125 and 1062 - 1034 cm−1 suggest the presence of -C-O-S; O=S=O;
,
and -S-O-H groups in the spectra of SU-PTE.
3.5. Adsorption of Metal Ions by PT, PTE, PTE Cation Exchange Resins and Bio-Rex 70 in Element Solutions
PTE consists of several polyphenol/catechin units hosting multiple ?OH groups that yield to modification at suitable conditions. Modification can be achieved through a variety of processes including, physical and chemical techniques such as crosslinking, substitution and incorporation of new functional groups. The incorporated chemical groups produced new exchange sites on PTE and improved the capacity of the produced resins to adsorb metal ions from the different solutions. The percentages of Fe2+, Ni2+, Pb2+, Cu2+ and Zn2+ removed by PT, PTE, the resins and Bio-Rex 70 are presented on Table 4. The result shows that apart from Bio-Rex 70, PTE resins developed through chemical modification with POCl3were more effective in removing nearly all the metal ions in the solutions in contrast to that obtained through functionalizing alone.
As a bi-functional reagent, POCl3 reacts with -OH group containing molecules in two distinct ways to produce two different products. It crosslinks or substitutes an -OH group in a compound to form a product which contains mono-anion or di-anion
groups. The presence of these groups on the surfaces of
modified PTE cation exchange resins and the absence of the di-anion on the surface of that modified with CSA alone explains the disparate adsorption efficiency in favour of PS-PTE, Crosslinked-PPTE and Substituted-PPTE over SU-PTE.
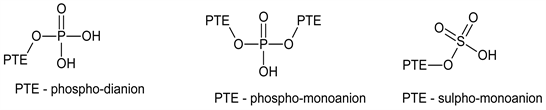
Among the POCl3-modified resins, PS-PTE demonstrated the highest metal ion adsorption ability over Crosslinked-PPTE and Substituted-PPTE and this is a consequence of the combined presence of the
and
groups in the resin molecule. In addition to high polar nature of both groups, the presence of lone pair electrons on the O=S=O and -P=O bonds increased the electron density around the groups and thereby increased the affinity of the groups for the positive metal ions in the solutions. References [29].30].31].bserved
![]()
Table 4. Percent removal of Fe2+, Ni2+, Pb2+, Cu2+ and Zn2+ by PT, PTE, PTE resins and Bio-Rex 70 in element solutions.
enhanced metal ion binding capacities demonstrated by bi-functional phosphosulphonated cation exchange resins reportedly synthesized via different techniques.
Branching and the presence of plurality of
pendants due to created -C-O-P- bonds in crosslinked phosphorylated wood pulp against uncrosslinked phosphorylated wood pulp was explained [17].s reasons for the observed higher adsorption of the different metal ions by the former rather than the later. The intensities of the -C-O-P- absorption bands captured from the FTIR spectra of Crosslinked-PPTE and Substituted?PPTE in the current study are10.7 and 4.2 respectively, signifying a larger than 2:1 ratio of the presence of -C-O-P- bonds between the two products. This difference in the intensities of the -C-O-P- absorption bands in the spectra of the two resins accounts for the superior performance of Crosslinked-PPTE as an adsorbent for the investigated metal ions to Substituted-PPTE.
As electronegativity increases across a period in the periodic table of the elements, higher electronegative S (2.5) should more readily attract positively charged metal ions to form metal-S- bonds than would P (2.1) under similar conditions to form metal-P- bonds. Moreover, the intensity of the O=S=O absorption band at 1420 cm−1 which was found to be higher than that of the -P=O at 1246 cm−1 indicatesthat more -S=O groups attached to the PTE catechin units in the
monoester than -P=O groups attached to the polyphenol units in the phosphorylated resin. Notwithstanding, the presence of 3 electron withdrawing/seeking groups, i.e. 2 -OH and a unit -P=O groups on Substituted-PPTE empowers it to attract and hold on to higher amounts of the positively charged metal ions in the solutions than SU-PTE. Therefore, Substituted-PPTE, a PTE-di-anion seized and eliminated more of the metal ions in the solutions than SU-PTE, a PTE-mono-anion.
Furthermore, the effectiveness of PTE cation exchange resins derived through modification using POCl3 or CSA alone or both POCl3 and CSA remarkably followed the trend: CSA < Substituted-PPTE < Crosslinked-PPTE < PS-PTE suggesting a correlation between the ion exchange capacity (IEC) of a resin and the type and magnitude of the ionisable groups on it. The findings from the current research validate that reported [17].bout higher Zn2+, Pb2+, Fe2+, Ni2+ and Cr2+ adsorption efficiencies by phosphosulphonated over phosphorylated crosslinked, phosphorylated but not crosslinked and sulphonated corncobs.
All the modified resins showed to remove higher amounts of the metal ions in the solutions than PT and PTE because, in the case of Crosslinked-PPTE,the adsorption capacity is a summation of the adsorption capacities of individual polyphenol/catechin units which joined to form it. Chemical modification of an adsorbent improves the uptake of metal ions by increasing the number of accessible sites on the adsorbent, imparting superior ion exchange properties and enhancing its adsorption capacity. The enhanced metal ions adsorption demonstrated by modified PTE polyphenols against the starting materials is ascribed to the different functional groups, including, -P=O, -C-O-S, O=S=O,
, S-OH,
, -P-OH, O=P-OH which were incorporated on testa. The groups increased the acidity of the adsorbate solutions and in synergy with the unreacted -OH andphenolic (-C6H5) groups of PTE polyphenols contributed to the relatively higher affinity of the synthesized cation exchange resins for the different metal ions. These, in addition to increased polarity and electronegativity of the added groups are presented for the outbalanced performances of the modified PTE adsorbents over the starting materials.
Furthermore, PT, PTE and the cation exchange resins observably removed the metal ions in a particular trend. Apart from PS-PTE and the starting materials, PT and PTE which removed more Cu2+ than Zn2+, Ni2+ than Fe2+ and Pb2+ than Ni2+, Fe2+ > Ni2+ > Pb2+ > Zn2+ > Cu2+ was the order by which the rest of the modified resins eliminated the metal ions in the solutions. Catechin is known for its excellent chelate formation with Fe. Reference [32].eported the influences of electrical charge and ionic radius as well as electronegativity of a metal and stearic conformation of the resulting metal-ion-resin-complex on the IEC, metal ion affinity of different functional groups and the amount of the metal ion in a solution which can be removed by a cation exchange resin. Substantiating, reported preferential adsorption of Hg2+ above Cd2+ and Pb2+ by 6-poly-dimethylaminoethyl-methacrylate-rice-straw (PDMAEMRS) grafted copolymers attributable to the former’s atomic size, ionic charge and interaction with other constituents of the solution.
The ionic radius of an element conveys the extent of its ionic size or the mean distance from the centre of its nucleus to the boundary of the closest electron cloud. Fe2+, Ni2+, Zn2+, Cu2+ and Pb2+ respectively are characterized by ionic radii of 0.65 Å, 0.69 Å, 0.74 Å, 0.73 Å and 1.19 Å. Besides Pb2+, Fe2+, Ni2+, Zn2+ and Cu2+ belong to the 4th period in the periodic table of the elements and although individual ionic radii do not significantly differ from one another, progress of the order: Fe2+ < Ni2+ < Cu2+ < Zn2+ obtains. While others [10].ttributed the favourable adsorption of Mg2+ over Cd2+, Zn2+, Cu2+ and Pb2+ by alkaline treated 2, 4-dichloro-6-(phenoxy-4-sulphonic acid)-1,3,5- triazine-orange mesocarpextract resin to the small ionic size of Mg2+, the higher affinity for Cu2+ of smaller ionic size, demonstrated by -OH and -SH ligands in untreated and acid treated cassava (manihot esculentacranz) waste was acknowledged by other reports [33].or its preferential adsorption from solution over Zn2+. Likewise, the smaller ionic sizes of Fe2+ and Ni2+ relative to the other ions are presented for the preferential adsorption of the duo from the solutions by the PTE cation exchangers under investigation.
Although the removal of the metal ions by PTE and its derivatives seemingly followed a particular trend, PS-PTE and Crosslinked-PPTE demonstrated the highest Pb2+ adsorption while the converse was the case by PT and PTE. In addition to enhanced capacity imparted through chemical modification, report [34].ndicates that affinity of
resins for metal ions is highest for ions of large ionic radii like Pb2+ may explain the remarkable Pb2+ binding capacity observed for PS-PTE. In the case of Crosslinked-PPTE, the amount of Pb2+ removed was basically a demonstration of the combined adsorption strengths of the individual PTE monomer units in the polymer.
The results also showed that PT removed more of the metal ions than PTE. This is attributed to the difficulty of the large ionic radius Pb2+ to migrate, attach and secure to the binding sites of jelly PTE while the other metal ions of comparatively smaller sizes held only loosely, whereas, all bound to PT, albeit, little (relative to the amounts bound by the modified PTEs) by a mutual strength of the rough macroporous and smooth jelly polyphenols.
Notwithstanding, PTE polyphenols was extracted before modification to downplay the association between the different biomass constituents reported [35].or the observed comparatively little integration of chemical groups on banana leaves.
3.6. Adsorption of Metal Ions in a Multi-Elements Solution and the Effect of Competing Metal Ions in Solutions of Low and High Metal Ions Concentration
Industry wastewater and discharges contain several heavy metal ions as pollutants. It is essential to evaluate the adsorption of each pollutant in real world situations. In such circumstances, metal ions in a solution compete with one another for the limited adsorption sites on an adsorbent’s surface with the preference of the adsorbent for individual metal ions remotely sufficient to suggest the amount of a metal ion that would bind to the particular adsorbent under competitive conditions. Not even the metal complexation and chelation prospects of an adsorbent can confidently describe its behaviour in a multi-element system challenged by interplay between metal ions competing for binding sites on an adsorbent. Adsorption of heavy metal ions in a multi-element system depends not only on adsorbent surface properties and adsorbate solution chemistry and properties, but on the magnitude as well as type and strength of the different metal ions competing for the active sites on the adsorbent. Adsorption capacity of an ion exchange resin (IER) for every contaminant heavy metal in an industry waste stream must be established before selecting a particular resin if optimum performance is to be achieved. The results of the adsorption of Fe2+, Ni2+, Pb2+, Cu2+ and Zn2+ in a multi-elements solution and the preference of modified PTE cation exchange resins and Bio-Rex 70 for a metal ion over another in a competitive environment is presented on Table 5 while the graphical illustration of the comparison of the affinity of PS-PTE resin for the metal ions in multi-elements solutions of low and high metal ion concentration is shown in Figure 2.
Although the trend was similar to that in the element solutions, the modified PTE resins performed poorly and demonstrated little competencies in removing the metal ions in the multi-elements solution. The metal ions competed with one another in the multi-elements solution during the adsorption process. Whereas 84.4 and 48.6; 79.8 and 46.7; 83.1 and 48.6; 71.7 and 41.1 and 81.8 and 51.8 percentages of Fe2+ respectively were bound by Crosslinked-PPTE,
![]()
Figure 2. Comparison of % removal of Fe2+, Ni2+, Pb2+, Cu2+ and Zn2+ by PS-PTE in multi-elements solutions of low and high metal ions concentrations.
![]()
Table 5. Percent removal of Fe2+, Ni2+, Pb2+, Cu2+ and Zn2+ by modified-PTE resins and Bio-Rex 70 in a multi-elements Solution.
Substituted-PPTE, PS-PTE, SU-PTE and Bio-Rex 70 in individual and multi-elements solutions, 97.2 and 26.3; 92.4 and 23.5; 96.8 and 16.6; 96.3 and 21.7 and 91.6 and 22.1% of the same were removed by PS-PTE in competitive environments of low and high metal ions concentration.
The results showed that the modified PTE resins demonstrated near equal capacities in removing the metal ions in the multi-elements solution. The effect of the competitive environment which showed to be relatively mild on Fe2+ significantly impacted Zn2+ which demonstrated the least adsorption on the resins. The variance between the amounts of a metal ion taken up by an adsorbent in the individual element solution and that in the multi-elements type can be attributed to reduction in the numeral of binding sites on the adsorbent which are available for occupation to the metal ion at the prevailing experimental conditions due to competition for the same sites with complementary ions present in the multi-elements solution. The number of sites available for occupation decreased due to overcrowding and blocking of adsorbent sites by the stampeding metal ions. Reference [36].37].eported comparatively lower adsorption capacities for metal ion adsorbents and drastic reductions in the amounts of Cu, Zn and Pb adsorbed from a ternary mixture by HNO3 treated oil palm root in contrast to the amounts of the metal ions removed from individual element solutions using the same adsorbent. Report [38].stablished the governance of the occupation of the limited binding sites on banana pith by equilibrium competitions between contending metal ions in the adsorbent-adsorbate mixture. The adsorption sites on the surfaces of the modified PTE resins in this study were contested for, occupied and saturated by metal ions with superior propensity against the susceptible ones. Consequently, adsorption sites partitioned and metal ions with greater and more favourable tenacity in terms of smaller ionic radii diffused freely and occupied the sites faster and more readily than the disadvantaged ones.
Whereas most of the metal ions in the multi-elements solution that contained 5 ppm of the individual metal ions were absorbed by PS-PTE, the amounts of the same metal ions removed from that containing 100 ppm of the ions were much reduced. This is as a result of the availability of adsorption sites for occupation to the metal ions in the multi-elements solution of low metal ions concentration, which relatively, was a dilute solution while the metals ions in the solution of high metal ions concentration, reasonably, a concentrated solution scrambled for few available sites. Competition was therefore less severe in the multi-elements solution of low metal ions concentration and vice-versa. This implies that the modified PTE adsorbents in this study would perform better and remove larger amounts of pollutant metal ions in wastewater if the concentrations of the metal ions are not so high.
3.7. Cation Exchange Capacity of PT, PTE, PTE Cation Exchange Resins and Bio-Rex 70
Ion exchange capacity (IEC) describes the ability of an insoluble polymeric material in a solution to replace flexible ionic charges in its structure with ions of opposite charge in the same solution with it. It is a crucial feature of all ion exchange resins; cationic or anionic alike and is expressed in terms of equivalents per litre in a liquid resin or milli-equivalents per gram in a dry powder. The CEC of a substance/polymeric material expresses its ability to exchange loosely bound positive ions in its structure with others in the same environment with it and is a pointer to the number of electrical charges of opposite sign on the material’s surface. Other reports [26].quated its magnitude to the amount of polar functional groups integrated to the chemical structure of a biological polymer in a modification process. Another report [39].xpressed it as the equivalence to the number of H+ released by a cation exchange resin flooded by a solution of a neutral salt. CEC of the PTE resins in this study was estimated by exchanging Na+ for H+ (liberated during HNO3 activation or conversion of the resins to the H+ forms) as a solution of Na2SO4 streamed through the resin and ranged between 4.23 and 9.52 meq/g [25]. This implies that Crosslinked-PPTE, Substituted-PPTE, PS-PTE and SU-PTE possessed higher CECs and accordingly higher numbers of replaceable H+ than the starting materials.
3.8. Point of Zero Charge (pH(pzc)) of PT, PTE and PTE Resins
Point of zero charge at different pH was determined for the individual resins in order to understand the surface properties of the PT adsorbents, hence, pH at zero point charge (pH(zpc)). The pH(ZPC) of an adsorbent is an essential property that communicates the pH at which there are no electrical charges on its surface. Defined as the point, on the pH scale at which the properties of the adsorbent-adsorbate suspension is unaffected by the surface characteristics (acidic or basic) of the adsorbent, it is the point on the graph where the arc of the plot of the variance between the initial and final pH (ΔpH = pHi − pHf) of the suspension against the initial pH i.e. ΔpH verses pHi intersect and corresponds to pHi − pHf; thus, ΔpH = pHi − pHf = 0. The pH(zpc) of PT, PTE, Crosslinked-PPTE and Substituted-PPTE as well as PS-PTE and SU-PTE reported [25].re all less than the pH of the adsorbate solution (pH = 6 ± 0.5), which explains their ability to remove metal ions in an aqueous solution. Since an adsorbent’s surface characteristically saturates with groups of comparable, rather than complementary pH, Crosslinked-PPTE, Substituted-PPTE, PS-PTE and SU-PTE possess the capacity to operate over a wide pH range with individual resin surface hosting positive or negative charges respectively as the pH of the solution fluctuates below or above its pH(pzc).
The functional groups (-SO3H, -P=O, -P-OH, -C-O-S and O=S=O) incorporated on PTE by the modification brought about a reduction in the pH(pzc) of all the products. Individual resins, therefore, attracted the positively charged metal ions at a pH where negative charges converged at its surface. Metal ion adsorption capacity of an adsorbent improves by increased pH of the surrounding solution, the effect of which, the presence of negative charges on the adsorbent’s surface is boosted. Reference [40].xplained that maleic acid modified Babasuepicarp (BEM) readily attracted positive charges after the pH of the adsorbate solution surpassed the pH(pzc) at 6.45 because the formation of negative chargeson the surface of BEM, which was essential for metal ion adsorption was only feasible at that pH. As the magnitude of negative charges disproportionate between pHi and pH(pzc) in favour of pHi, all the modified PTE resins removed more metal ions in line with the size difference between its pH(pzc) and pH of the solution at the commencement of the experiment. The increase in the amount of a metal ion removed climaxed at pH 8.0 and thereafter diminished until equilibrium due to the formation of an adsorbent-metal complex that shielded the adsorbent surface and restricted interaction between the resin and the metal ion in the solution.
4. Conclusion
The use of peanut testa for wastewater purification demonstrated in this study illustrates gainful ways to transform this waste that has defaced the landscape in many cities into potential strong cation exchange resins.
Acknowledgements
The authors are grateful to the World Bank African Centre for Oilfield Chemicals Research for partially funding the research. Also, the Management and Staff of Getamme Laboratory Services, Port Harcourt for providing the facilities for the experiments and the Management and Staff of Idex Scientific Supplies Company for providing the chemicals.