1. Introduction
Organic compounds containing isoxazolines and pyrazolines scaffold as a core unit, are known to exhibit various biological and pharmaceutical activities [1] such as antiviral [2], antibacterial [3], antitumor [4], anti-inflammatory [5], antifungal [6]. Owing to diverse biological properties, various isoxazolines and pyrazolines derivatives have gained much more attention in the field of synthetic and medicinal chemistry [7]. Among it, isoxazolines and pyrazolines pharmacores are an important class of five-membered nitrogen-oxygen, nitrogen-nitrogen containing heterocyclic compounds that are widely distributed and exhibit diverse biological properties of great significance [8].
The most common synthetic methods of isoxazolines are cycloaddition reactions, with the 1,3-dipolar cycloaddition of nitrile oxides with alkenes [9], in addition to radical cyclization reactions, classes of cyclization (5-exo-trig) in this strategy yield cyclic products via radical intermediates. They usually proceed in three basic steps: selective radical generation, radical cyclization, and conversion of the cyclized radical to product [10]. The most common methods are used to synthesize isoxazolines branched from these two strategies. Isoxazolines are also indispensible building blocks in organic synthesis [11]. Usually, the 2-pyrazolines construction can be built through the following four ways: 1) cycloaddition reactions of hydrazines with α, β-unsaturated aldehydes or ketones [12], 2) microwave-assisted cyclocondensation reactions between alkyl dihalides and hydrazines [13], 3) 1,3-dipolar cycloaddition of diazoalkanes or nitrilimines with alkenes [14], and 4) halocyclization of β, γ-Unsaturated hydrazones [15] [16]. However, the relatively high reaction temperature and the finite substrate range of methods a and b, to some extent, limit their wide application in synthetic chemistry, while method c sometimes leads to regioisomers mixing, due to the regioselectivity of poverty in some cases of cycloaddition process. Therefore, it is still advisable to develop new and more effective ways to build diversity instead of 2-pyrazolines [15]. 2-Pyrazolines proved to be the most useful pyrazoline type of compounds. As generally used simple and convenient procedure is based on the reaction of β, γ-unsaturated compounds with chlorine sources [16]. Halogen promoted addition of nucleophiles to alkenes represents one of the most fundamental reactions in chemistry and is widely used in organic synthesis for the stereoselective introduction of functional groups. Trichloroisocyanuric acid (TCCA) is used as an innocuous and versatile oxidant or chlorinating reagent with high stability in industry [17]. N-Chlorosuccinimide (NCS) is generally used for chlorination reactions or certain mild oxidations [18], in addition to his chlorinating and oxidizing agent that is used as source for chlorine in radical reactions and various electrophilic additions. In synthetic organic chemistry, TCCA has been present in a variety of reactions due to its low price, ready availability, environmentally benign attributes, and serving as a source of three chlorine atoms for a variety of reactions [19].
Herein we will report an efficient chlorocyclization of β, γ-unsaturated oximes with NCS and β, γ-unsaturated hydrazones with TCCA, then test other halocyclization of both of β, γ-unsaturated compounds with different halosources to yield 2-isoxazolines and 2-pyrazolines.
2. Experimental Results and Discussion
We commenced by the oxychlorination of allylic oximes. At the outset, the reaction parameters which provided a concise and efficient method for preparation of chloro-substituted 4, 5-dihydroisoxazoles, were systematically surveyed. It was found that using NCS, serving as both chlorine source and oxidant for the redox cycle, gives good results for reactions. Among the solvents examined, from the data in Table 1, we examined the effect of solvents (such as acetonitrile, toluene, dichloromethane, tetrahydrofuran, methanol, and mixed solvents of dichloromethane and toluene) on the yield of the reaction (Table 1), the results
![]()
Table 1. Optimization of reaction conditionsa.
aAll reactions were carried out by using 0.2 mmol of 1a in 5 mL of solvent.
showed that acetonitrile can be used as the best solvent. We tested the effect of the amount of reactants on the reaction (Table 1) when the ratio of NCS 2 and oxime 1a was 1: 2, the yield was increased to 97% with the change of reactions time to 24 h. There is no addition of inorganic or organic additive to the reaction, about 26 reactions were done 20 reactions succeeded and gave good yield (67% - 98%) and 5 reactions had no yield.
In Table 2 we compare the halocyclization of β, γ-unsaturated oxime with NCS which is completed in 10 h in air atmosphere with the presence of CH3CN as solvent and (2 equiv) ratio NCS to yield 92% of 2-isolxazoline 3, and halocyclization of β, γ-unsaturated oxime 1 with NBS which need only 20 minutes to complete at the same condition with yield 95%. However, the replace of NCS with TCCA directed the reaction to the interaction to complete in 6 h under the pressure of N2 with 0.6 equiv to yield 87% of compound 3, according to optimization we found that chlorocyclization of β, γ-unsaturated oximes with NCS is better than chlorocyclization of it with TCCA.
With the optimal reaction conditions in hand, we next explored the scope of the reactions with a variety of β, γ-unsaturated oximes 1. As shown in Scheme 1, the electronic and steric properties has some effect on the product so in the aryl substrates ortho substituted (3d, 3g) gave low product compared with the same aryl substrates substituted para and meta (3b, 3c) for methyl group and (3i, 3h) for chloride group, (3i) obtained the highest yield 98%, most of the reactions
![]()
Scheme 1. Substrate Scope of Reactiona. aAll reactions were carried out using 0.2 mmol of 1a, 2 equiv of NCS, and 8 mL of CH3CN.

![]()
Table 2. The exam of different halocyclizaion.
aIsolated yields.

completed in about 18 hours but some of them completed in 24 hours in addition to this reactions, aryl substrates substituted in para position with chloride took only 6 hours and that substituted in para position with bromide took about 10 hours, all compounds obtained at the room temperature with the solvent CH3CN and N-chlorosuccinimide (NCS) only without any catalysts, the aryl substrates substituted with nitro or amine (3q, 3v, 3r) and substituted furan (3t), didn’t give a result in the reaction with the NCS. This method can apply over wide range of aliphatic and aromatic oxime (3z) to produce 2-isoxazoline but the yield of chlorocyclization of aromatic oxime is higher than the yield of chlorocycilzation of aliphatic oxime.
The development of atom-economic and direct synthetic methods to these heterocycles has received considerable attention, therefore, the exploration of new strategies and reagents to develop more efficient methodologies for the rapid assembly of N-heterocycles is highly desirable [19]. Recently N-centered radicals generated direct transformation of the N-H bond into an N-centered radical [20]. It was found that by using TCCA, serving as both chlorine source with the β, γ-unsaturated hydrazones and oxidant for the redox cycle to produce 2-pyrazoline, gives good result for reactions. We initially tested the feasibility of cyclization of β, γ-unsaturated hydrazone dz1 with TCCA dz2 under nitrogen atmosphere N2 with the mole ratio 1/0.5 in the presence of solvent CH3CN at room temperature and it produced 36% yield (Table 3, entry 1). After brief optimization of mole ratio of the reactants, we found that the mole ratio of 1/0.65 for dz1/dz2 is the best ratio to give the product dz3 in good yield 92%. In the

![]()
Table 3. Optimization of reaction conditionsa.
aAll reactions were carried out by using 0.2 mmol of dz1 at the room temperature.
absence of nitrogen atmosphere N2 there is no reaction and the yield% is 0% (Table 3, entry 4), the amount of TCCA was increased to 0.65 equiv and the yield of dz3 amounted to 92% (Table 3, entry 3). Regrettably, the yield of dz3 wasn’t elevated again when the amount of TCCA was further increased (Table 3, entry 5). And in the absence of N2 (also examined in the reaction) the yield of the product was not improved (Table 3, entry 4), also the optimization of other reaction conditions (Table 3, entry 6) when NaOAc was added, the yield was decreased to only 20%. For the addition of Cu (OAc)2 (Table 3, entry 7) the reaction needed 48 h to give 60% of the product dz3. The addition of CuCl (Table 3, entry 8) yielded only trace. We examined the effect of some solvents such as acetonitrile, dichloromethane and tetrahydrofuran, the results showed that solvent acetonitrile can be used as the best candidate. By using solvents CH2Cl2 and THF, and additive CuCl and Cu (OAc)2, the result obtained was 0% yield (Table 3, entries 9 - 12). The optimization in Table 3 showed this reaction to be free metal, no additives needed in mild reaction.
In Table 4 we compare the halocyclization of β, γ-unsaturated hydrazones dx1 with TCCA which is completed in 6 h under the pressure of N2 with 0.65
![]()
Table 4. Examine of defferent halocyclizaion.
aIsolated yields.

equiv to yield 90% of compound dx3 with the presence of CH3CN as solvent, and halocyclization of β, γ-unsaturated hydrazones dx1 with NBS which need only 4 h to complete under air atmosphere to yield 94%, however the change of TCCA with NCS lead the reaction to complete in 24 h under air atmosphere with 4 equiv of NCS to yield 80% of compound dx3. According to optimization we found that chlorocyclization of β, γ-unsaturated hydrazones with TCCA is peter than chlorocyclization of it with NCS.
β, γ-unsaturated hydrazones dz1 bearing electron-withdrawing groups such as Cl, Br, F, CF3 in the para and meta positions underwent smooth cyclization to furnish the desired products dz3 higher yields (2z, 3z, 11z, 13z, 28z, 29z, 32z, 33z). With further investigation we found that hydrazones dz1, with aryl para position substituted, showed high reactivity more than meta position substituted, on the other hand, ortho position substituted furnish the desired products in slightly lower yields (5z, 9z, 12z). β, γ-Unsaturated hydrazones dz1 with electron-donating groups in both the para and meta position gradually provided the corresponding pyrozilines dz3 from good to excellent (6z, 10z, 30z). The electronic and steric properties have some effect on the product, such as hydrazone dz1 with aryl substrates ortho and para position substituted (19z), which showed the lower yield to be 70%, in addition to, hydrazones dz1 with aryl substrate with the methoxy substituted in meta position gave only 78% yield (4z), other different hydrazone dz1 substituted CN in para position gave good yield 89% (7z). Hydrazones dz1 with nitro and carboxylic group substituted and hydrazone pyridin also examined in the reaction, yielded no product improvement (17z, 16z, 26z). When the hydrazones is N’-(1-phenylbut-3-en-1-yl) benzohydrazide, the reaction underwent cyclization smoothly to furnish the desired products dz3 provided higher yield as was mentioned, but when it was phenyl 2- (1-phenylbut-3-en-1-yl) hydrazinecarboxylate or N'-(1-phenylbut-3-en-1-yl) benzenesulfonohydrazide, yield of the product was not improved (20z, 22z, 23z, 24z, 25z), the thiophen hydrazone and phenylpropyl hydrazone gave good yield (8z, 15z). This method can apply over wide range of aliphatic and aromatic hydrazones (3x) to produce 2-pyrazolines but the yield of chlorocyclization of aromatic hydrazones is higher than the yield of chlorocycilzation of aliphatic hydrazones (see Scheme 2).
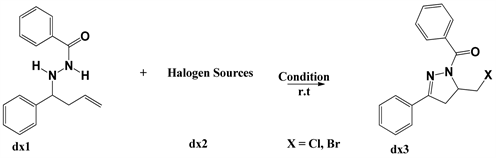
Hydrazone dz1 (0.2 mmol, 1.0 equiv.), TCCA dz2 (0.13 mmol, 0.65 equiv.), loaded into a flame-dried flask, anhydrous CH3CN (8 mL) was added to the mixture, and the mixture was then stirred at room temperature under the N2 pressure until the starting material had been consumed and determined by TLC. The mixture was then extracted with ethyl acetate (3 * 15 mL). The combined organic extracts were washed with brine, dried with Na2SO4, filtered, concentrated, and purified by flash chromatography on silica gel (ethyl acetate/petroleum ether 1/4) to give product (dz3).
Illustrated in Scheme 3, when β, γ-unsaturated compounds c1 was stirred with NCS or TCCA produced radical 2a, which abstracted a proton from the hydroxyl group in oxime 1a to produce radical e1. NCS or TCCA was finally converted into succinimide and cyanuric acid gradually, which was detected from the reaction mixture. Cyclization of radical e1 gave another carbon radical f1, which reacted with chloride radical to give the final product g1.
3. Conclusions
In summary we have developed TCCA/NCS-promoted cascade chlorination and cyclization of β, γ-unsaturated compounds, a vast array of chlorinated 2-isoxazolines and 2-pyrazolines has been divergently synthesized in moderate to good yields. Acetonitrile is used as solvent, according to optimization of reaction conditions. We found that trichloroisocyanuric acid (TCCA) is the best to use as a chlorinated of 2-pyrazolines and N-chlorosuccinimide (NCS) is best to use as a chlorinted of 2-isoxazolines. 2-Isoxazolines and 2-pyrazolines were obtained in good to excellent yields (67% - 98%) without any additives under mild reaction conditions.
3.1. General Information
Unless otherwise noted, all reactions were carried out under an air atmosphere at room temperature, materials were purchased from commercial suppliers and used without further purification. All solvents were purified and dried according to standard methods prior to use. 1H NMR and 13C NMR spectra were recorded on a Varian instrument (600 MHz and 150 MHz) spectrometer in CDCl3 using
![]()
Scheme 2. Substrate Scope of Reactiona. aAll reactions were carried out using 0.2 mmol of dz1, 0.65 equiv of TCCA dz2, and 8 mL of CH3CN. All reaction undergoes N2 atmosphere in room temperature. Isolated yields.
tetramethyl silane (TMS) as internal standard. Data for 1H NMR were recorded as follows: chemical shift (δ, ppm), multiplicity (s = singlet, d = doublet, t = triplet, m = multiplet, coupling constant (s) in Hz, integration). Data for 13C NMR is reported in terms of chemical shift (δ, ppm). High resolution mass spectra (HRMS) were obtained by the ESI ionization sources.
3.2. Synthesis Procedure
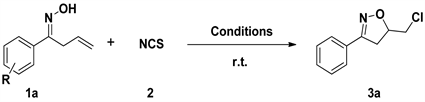
Oxime 1a (0.2 mmol, 1.0 equiv.), NCS 2a (0.4 mmol, 2 equiv.), loaded into a flame-dried flask, anhydrous CH3CN (8 mL) was added to the mixture, and the mixture was then stirred at room temperature until the starting material had been consumed as determined by TLC. The mixture was then extracted with ethyl acetate (3 * 15 mL). The combined organic extracts were washed with brine, dried with Na2SO4, filtered, concentrated, and purified by flash chromatography on silica gel (ethyl acetate/petroleum ether 1/20) to give products (3).
Acknowledgements
The authors are greatly indebted to the anonymous referees for their careful reading and helpful comments, especially the referee who gave constructive suggestions for revision of the manuscript. We are thankful for financial support from the National Natural Science Foundation of China.