Design, Synthesis and Antibacterial Activity Evaluation of 4,5-Diphenyl-1H-Imidazoles Derivatives ()
1. Introduction
Infectious diseases caused by bacterial pathogens represent a major public health issue in recent years due to the emergence and spread of new strains of bacteria [1] and the widespread occurrence of drug resistance [2]. Thus, the development of new types of antibacterial drugs, especially those with a new drug target and/or with the ability to overcome drug resistance [3] is an urgent need. Structural modification of antimicrobial drugs to which resistance has developed, has been shown to be an effective way to prolong the lifespan of antifungal agents such as azoles [4], antiviral agents such as nonnucleoside reverse transcriptase inhibitors [5], and various antibacterial agents including imidazole and benzimidazole [6] [7] [8] [9]. Currently, some of the various marketed drugs available (Figure 1) have imidazole or benzimidazole in their structures.
Because the heterocyclic ring includes the core of the active moiety or pharmacophores, we have been interested in working on structural modification of imidazole and benzimidazole. Besides this, these two heterocyclics containing nitrogens can be substituted in different positions to give several compounds with antibacterial activity [10] [11].
Considering the importance of both moieties imidazole and benzimidazole, we hypothesize their presence in the same structure would lead to compounds with higher potent. In the present study, we report the design, synthesis and antibacterial activities of some compounds containing both imidazole and benzimidazole motifs (Scheme 1 and Scheme 2), modified from 4,5-diphenyl-imidazol -2-thiol. All the structures of the synthesized compounds were confirmed by NMR (1H and 13C) and HRMS spectra and their activities were illustrated against some gram-positive and gram-negative bacteria.
2. Materials and Methods
2.1. Chemistry
All chemical reagents and solvents used were of reagent grade or purified using standard methods. NMR spectra were recorded at 1H (400 MHz or 600 MHz) and 13C (101 MHz or 400 MHz) on a Bruker instrument. Coupling constants (J) and chemical shifts (δ) are given in hertz and ppm respectively, using TMS (1H NMR) and solvents (13C NMR) as internal standards.
High resolution mass spectrometry (HRMS) analyses are performed on a hybrid quadrupole time-of-flight mass spectrometer (Micromass-Waters Q-TOF Ultima global), equipped with an electrospray ionization source and a MALDI source. All these analyses are carried out at the Laboratory of Glycochemistry, Antimicrobials and Agroresources (LG2A) of the University of Picardie Jules Verne, Amiens. The melting points were measured using a KOFLER bench.
The purification of the products is carried out by flash chromatography on
![]()
Figure 1. Chemical structure of some imidazole and benzimidazole-supported anti-infective compounds.
silica gel (Silica gel 60) pushed by compressed air using a mixture of cyclohexane/ethyl acetate eluent in variable proportions. Thin layer analytical chromatography (TLC) was performed on silica gel on aluminum support (Merck DC-Autofolien Kiesegel 60 F254 commercial plates, silica thickness 0.2 mm in normal phase). The products are then revealed under UV lamp (wavelength 254 nanometer).
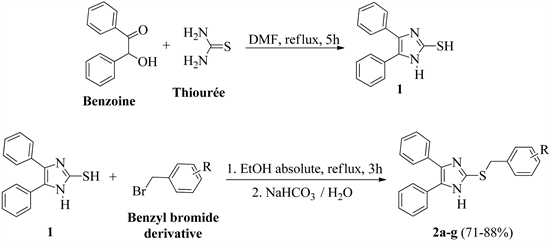
General procedure for the synthesis of 2-(benzylthio)-4,5-diphenyl-1H-imidazoles 2a-g
The synthesis of 2-(benzylthio)-4,5-diphenyl-1H-imidazoles 2a-g was carried out in two steps from benzoin. First, 4,5-diphenyl-1H-imidazol-2-thiol 1 was prepared by the condensation of benzoin with thiourea in dimethylformamide (DMF) at 150˚C using the reported procedure [12]. Then, to a solution of 4,5-diphenyl-imidazol-2-thiol (50 mg; 0.1985 mmol) in absolute ethanol (10 mL), an appropriate benzyl bromide derivative (1.2 eq) was added. The mixture was refluxed for 3 hours. After cooling to room temperature, the reaction mixture was placed in an ice bath then neutralized with sodium hydrogen carbonate solution (5%). The mixture was diluted in ethyl acetate and washed several times with water. The organic phase was dried over anhydrous sodium sulphate, filtred and the solvent was evaporated under reduced pressure.
The residue obtained was purified by silica gel chromatography (cyclohexane/ethyl acetate: 30/70) to give the compounds 2a-g.
2-(benzylthio)-4,5-diphenyl-1H-imidazole 2a [13]
White crystals, yield = 85%, m p: 186˚C. 1H NMR (400 MHz, DMSO-d6) δ: 12.57 (s, 1H, NH), 7.49 - 7.19 (m, 15H, H-Ar) 4.40 (s, 1H, SCH2); 13C NMR (101 MHz, DMSO-d6) δ: 138.52 (C=N), 128.61 (2C, CH-Ar), 128.38 (2C, CH-Ar), 128.12 (2C, CH-Ar), 127.91 (2C, CH-Ar), 127.54 (2C, CH-Ar), 127.35 (1C, CH-Ar), 126.92 (1C, CH-Ar), 126.68 (2C, CH-Ar), 126.26 (1C, CH-Ar), 36.97 (SCH2). HRMS: m/z calculated for C22H19N2S [M + H]+: 343.1269; found: 343.1272.
2-(4-nitrobenzylthio)-4,5-diphenyl-1H-imidazole 2b
Yellow crystals, yield = 70%, m p: 168˚C. 1H NMR (400 MHz, DMSO-d6) δ: 8.13 (d, 2H, H-Ar), 7.46 - 7.42 (m, 6H, H-Ar), 7.33 - 7.28 (m, 6H, H-Ar), 4.34 (s, 1H, SCH2); 13C NMR (101 MHz, DMSO-d6) δ: 146.61 (NO2-C-Ar), 146.54 (CH2-C-Ar) 139.59 (C=N), 129.86 (4C, CH-Ar), 128.40 (1C, CH-Ar), 127.93 (1C, CH-Ar), 127.55 (1C, CH-Ar), 127.43 (1C, CH-Ar), 126.66 (1C, CH-Ar), 126.32 (1C, CH-Ar), 123.23 (4C, CH-Ar), 36.03 (SCH2). HRMS: m/z calculated for C22H18N3O2S [M + H]+: 388.1120; found: 388.1108.
2-(4-trifluorobenzylthio)-4,5-diphenyl-1H-imidazole 2c
White crystals, yield = 78%, m p: 182˚C. 1H NMR (400 MHz, DMSO-d6) δ: 12.58 (s, 1H, NH), 7.68 (d, 2H, H-Ar), 7.47 - 7.19 (m, 10H, H-Ar), 4.46 (s, 2H, SCH2); 13C NMR (101 MHz, DMSO-d6) δ: 143.31 (CH2-C-Ar) 138.98 (C=N), 129.67 (2C, CH-Ar), 128.67 (2C, CH-Ar), 128.20 (2C, CH-Ar), 127.82 (2C, CH-Ar), 127.69 (1C, CH-Ar), 126.94 (2C, CH-Ar), 126.58 (1C, CH-Ar), 125.26 - 125.22 (2C, CH-Ar), 36.24 (SCH2). HRMS: m/z calculated for C23H18F3N2S [M + H]+: 411.1143; found: 411.1154.
2-(4-chlorobenzylthio)-4,5-diphenyl-1H-imidazole 2d
Light yellow crystals, yield = 73%, m p: 150˚C. 1H NMR (400 MHz, DMSO-d6) δ: 12.58 (s, 1H, NH), 7.49 - 7.47 (m, 2H, H-Ar), 7.42 - 7.37 (m, 8H, H-Ar), 7.33 (td, 1H, H-Ar), 7.29 (t, 2H, H-Ar), 7.23 - 7.20 (m, 1H, H-Ar), 4.38 (s, 2H, SCH2); 13C NMR (101 MHz, DMSO-d6) δ: 139.24 (C=N), 130.73 (2C, CH-Ar), 128.67 (2C, CH-Ar), 128.34 (2C, CH-Ar), 128.20 (2C, CH-Ar), 127.82 (2C, CH-Ar), 127.66 (1C, CH-Ar), 126.95 (2C, CH-Ar), 126.56 (1C, CH-Ar), 36.12 (SCH2). HRMS: m/z calculated for C22H18ClN2S [M + H]+: 377.0879; found: 377.0880.
2-(3,5-dichlobenzylthio)-4,5-diphenyl-1H-imidazole 2e
White crystals, yield = 76%, m p: 178˚C. 1H NMR (400 MHz, DMSO-d6) δ: 12.60 (s, 1H, NH), 7.48 - 7.20 (m, 13H, H-Ar), 4.33 (s, 2H, SCH2); 13C NMR (101 MHz, DMSO-d6) δ: 142.91 (CH2-C-Ar), 138.59 (C=N), 128.67 (2C, CH-Ar), 128.20 (2C, CH-Ar), 127.81 - 127.80 (4C, CH-Ar), 127.71 (1C, CH-Ar), 126.95 (2C, CH-Ar), 126.65 (1C, CH-Ar), 126.61 (1C, CH-Ar), 35.92 (SCH2). HRMS: m/z calculated for C22H17Cl2N2S [M + H]+: 411.0489; found: 411.0488.
2-(4-fluorobenzylthio)-4,5-diphenyl-1H-imidazole 2f [13]
Yellow crystals, yield = 88%, m p: 160˚C. 1H NMR (600 MHz, DMSO-d6) δ: 12.59 (s, 1H, NH), 7.51 - 7.50 (m, 2H, H-Ar), 7.45 - 7.42 (m, 2H, H-Ar), 7.41 - 7.39 (m, 3H, H-Ar), 7.33 - 7.27 (m, 3H, H-Ar), 7.22 - 7.20 (m, 1H, H-Ar), 7.15 - 7.12 (m, 2H, H-Ar), 4.40 (s, 2H, SCH2). 13C NMR (400 MHz, DMSO-d6) δ: 162.15 and 160.54 (F-C-Ar), 139.44 (C=N), 130.86 (1C, CH-Ar), 130.81 (1C, CH-Ar), 128.65 (2C, CH-Ar), 128.19 (2C, CH-Ar), 127.82 (2C, CH-Ar), 127.62 (1C, CH-Ar), 126.97 (2C, CH-Ar), 126.54 (1C, CH-Ar), 115.21 (1C, CH-Ar), 115.07 (1C, CH-Ar), 36.13 (SCH2).
2-(4-méthylbenzylthio)-4,5-diphenyl-1H-imidazole 2g
White crystals, yield = 88%, m.p: 164˚C. 1H NMR (400 MHz, DMSO-d6) δ: 12.57 (s, 1H, NH), 7.51 - 7.50 (m, 2H, H-Ar), 7.40 - 7.37 (m, 4H, H-Ar), 7.33 - 7.27 (m, 5H, H-Ar), 7.21 (t, 1H, H-Ar), 7.12 (d, 2H, H-Ar), 4.37 (s, 2H, SCH2), 2.26 (s, 3H, CH3); 13C NMR (101 MHz, DMSO-d6) δ: 139,74 (C=N), 128.96 (2C, CH-Ar), 128.80 (2C, CH-Ar), 128.64 (2C, CH-Ar), 128.18 (2C, CH-Ar), 127.80 (2C, CH-Ar), 127.59 (1C, CH-Ar), 126.96 (2C, CH-Ar), 126.51 (1C, CH-Ar), 36.79 (SCH2), 20.68 (CH3). HRMS: m/z calculated for C23H21N2S [M + H]+: 357.1425; found: 357.1423.
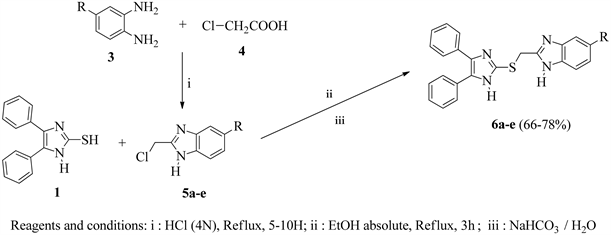
General procedure for the synthesis of 2-(chloromethyl)-1H-benzimidazoles 5a-e [14] [15] [16]
To a solution of orthophenylenediamine or derivatives 3 (50 mg; 0.1985 mmol) in 5 mL hydrochloric acid (4N), 1.5 equivalents of chloroacetic acid 4 was added. The mixture was refluxed for 15 hours. After cooling to room temperature, the reaction mixture was placed in an ice bath then neutralized with sodium hydrogen carbonate solution (5%). The mixture was diluted in ethyl acetate and washed several times with water. The organic phase was dried over anhydrous sodium sulphate, filtred and the solvent was evaporated under reduced pressure.
The residue obtained was purified by chromatography on silica gel (cyclohexane/ethyl acetate: 30/70) to give compounds 5a-e.
2-[(1H-benzimidazol-2-yl)methylthio]-4,5-diphenyl-1H-imidazole 6a [17]
White crystals, yield = 76%, m p: 152˚C. 1H NMR (400 MHz, DMSO-d6) δ: 12.92 (s, 1H, NH), 12.64 (s, 1H, NH), 754 - 7.32 (m, 12H, H-Ar); 7.18 (d, 2H, H-Ar); 4.62 (s, 2H, SCH2); 13C NMR (101 MHz, DMSO-d6) δ: 151.14 (C=N), 139.31 (C=N), 30.57 (SCH2). HRMS: m/z calculated for C23H19N4S [M + H]+: 383.1330; found: 383.1346.
2-[(5'-nitro-1H-benzimidazol-2-yl)methylthio]-4,5-diphenyl-1H-imidazole 6b
Solid, yield = 68%, m.p: 260˚C - 266˚C. 1H NMR (400 MHz, DMSO-d6) δ: 13.26 (s, 1H, NH), 12.78 (s, 1H, NH), 7.69 (d, 1H, H-Ar); 743 - 7.28 (m, 10H, H-Ar), 4.66 (s, 2H, SCH2); 13C NMR (101 MHz, DMSO-d6) δ: 156.94 (C=N), 138.52 (C=N), 30.69 (SCH2). HRMS: m/z calculated for C23H18N5O2S [M + H]+: 428.1181; found: 428.1177.
2-[(5'-trifluoromethyl-1H-benzimidazol-2-yl)methylthio]-4,5-diphenyl-1H-imidazole 6c
White crystals, yield = 70%, m.p: 220˚C. 1H NMR (600 MHz, DMSO-d6) δ: 12.88 (d, 2H, 2NH), 7.89 - 7.47 (m, 3H, H-Ar); 751 - 7.30 (m, 10H, H-Ar), 4.63 (s, 2H, SCH2); 13C NMR (400 MHz, DMSO-d6) δ: 138.72 (C=N, imidazole), 30.65 (SCH2). HRMS: m/z calculated for C24H18F3N4S [M + H]+: 451.1204; found: 451.1208.
2-[(5'-chloro-1H-benzimidazol-2-yl)methylthio]-4,5-diphenyl-1H-imidazole 6d
White crystals, yield = 66%, m.p: 248˚C. 1H NMR (600 MHz, DMSO-d6) δ: 12.80 (s, 2H, 2NH), 762 - 7.19 (m, 13H, H-Ar), 4.61 (s, 2H, SCH2); 13C NMR (400 MHz, DMSO-d6) δ: 152.58 (C=N), 138.95 (C=N), 30.55 (SCH2). HRMS: m/z calculated for C23H18ClN4S [M + H]+: 417.0941; found: 417.0959.
2-[(5'-methyl-1H-benzimidazol-2-yl)methylthio]-4,5-diphenyl-1H-imidazole 6e
White crystals, yield = 77%, m.p: 248˚C - 250˚C. 1H NMR (400 MHz, DMSO-d6) δ: 12.92 (s, 1H, NH), 12.48 (s, 1H, NH), 746 - 7.32 (m, 12H, H-Ar), 6.99 (d, 1H, H-Ar); 4.58 (s, 2H, SCH2), 2.40 (s, 3H, CH3); 13C NMR (101 MHz, DMSO-d6) δ: 151.44 (C=N), 139.38 (C=N), 30.57 (SCH2), 21.30 (CH3). HRMS: m/z calculated for C24H21N4S [M + H]+: 397.1487; found: 397.1480.
2.2. Antibacterial Assay
Antimicrobial tests of synthesized compounds 2a-e and 6a-e were evaluated against Pseudomonas aeruginosa and Escherichia coli (Gram-negative bacteria) and then against Staphyloccocusaureus and Enterococcus faecalis (Gram-positive bacteria). The method used was the liquid medium micromethod performed in a 96-well plate [18]. The compounds were dissolved in sterile DMSO so that their concentration in the wells of column 12 was 256 µg/mL followed by a dilution range of order 2 in DMSO to obtain a concentration series of the sample solution (from 256 to 0.063 µg/mL). After this dilution, 225 µL of Mueller-Hinton culture medium (previously prepared according to the supplier’s data) and 20 µL of the different inocula were added to 5 µL of the sample solution at different concentrations. Then the microplates were incubated at 37˚C for 24 hours. Absorbance measurements were performed at 600 nm with a plate reader. Bacterial growth results in a cloudiness in the well. Thus, if there is cloudiness in the well (Absorbance higher than 0.1), the bacteria has grown there; the compound has not allowed its inhibition (the compound has no antibacterial activity) at the corresponding concentration. In the opposite case, if there is absence of this disorder (Absorbance lower than 0.1), the compound has inhibited the growth of bacteria at the corresponding concentration (the compound has an antibacterial activity).
3. Results and Discussion
3.1. Chemistry
The synthetic route of the compounds 2a-g and 6a-e is outlined in Scheme 1 and Scheme 2 respectively. The synthesis of 2-(benzylthio)-4,5-diphenyl-1H-imidazoles 2a-g was carried out in two steps from benzoin. First, 4,5-diphenyl-1H-imidazol-2-thiol 1 was prepared by the condensation of benzoin with thiourea using the reported procedure [12]. Then compound 1 was coupled with an appropriate benzyl bromide derivative in absolute ethanol at reflux temperature to furnish after neutralization in the presence of NaHCO3 compounds 2a-g (Scheme 1).
The 2-(benzylthio)-4,5-diphenyl-1H-imidazoles 2a-g were obtained in good yields, from 71% to 85%, after purification by silica gel chromatography. The structures of all novel compounds were confirmed by 1H and 13C NMR spectra and mass spectrometry. The absence of the peak at 3.5 ppm attributed to the thiol proton (SH) in compound 1 and the appearance of the signals (protons and
Scheme 1. Synthesis of 2-(benzylthio)-4,5-diphenyl-1H-imidazoles 2a-g.
Scheme 2. Synthesis of 2-[(1-H-benzimidazol-2-yl)methylthio]-4,5-diphenyl-1H-imidazoles 6a-e.
![]()
Table 1. Melting points and yields of 2-(benzylthio)-4,5-diphenyl-1H-imidazoles 2a-g.
carbons) of the (SCH2) groups as well as the increase of the signals of the aromatic protons and carbons confirm the formation of compounds 2a-g.
The structures of synthesized compounds 2a-g and their physicochemical properties are given in Table 1.
Compounds 2-[(benzimidazol-2-yl)methylthio]-4,5-diphenyl-1H-imidazoles 6a-e were synthesized by coupling compound 1 with an appropriate 2-(chloromethyl)-1H-benzimidazoles 5a-e at refluxed temperature in absolute ethanol. Compounds 5a-e were obtained by condensation reaction between orthophenylenediamine derivatives 3 and chloroacetic acid 4 following a published procedure [19].
The 2-[(benzimidazol-2-yl)methylthio]-4,5-diphenyl-1H-imidazoles 6a-e were obtained after purification by silica gel chromatography in good yields, from 66 to 77%. Compounds 6a-e were characterized by 1H NMR, 13C NMR and HRMS. Their 1H NMR spectra indicate the disappearance of the signal at 3.5 ppm corresponding to the thiol proton (SH) in compound 1 and the appearance of new signals attributed to the protons of the (SCH2) group at 4.58 - 4.66 ppm. Chemical structures of synthesized compounds 6a-e and their physicochemical properties are given in Table 2.
3.2. Antibacterial Activity
The synthesized compounds 2a-e and 6a-e were evaluated for their in-vitro antibacterial activity against the gram positive Staphylococcus aureus and Enterococcus faecalis bacteria and the gram negative Pseudomonas aeruginosa and Escherichia coli bacteria. The MIC values were determined by using liquid medium micromethod performed in a 96-well plate. Ciprofloxacin was used as the reference antibacterial agents. All the biological results of the tested compounds are shown in Table 3. It was found that compounds 2a-e and 6a,b,e were not active against all bacteria tested at used concentrations. However two compounds 6c and 6d showed MIC values respectively at 16 µg/mL against gram positive bacteria (Staphylococcus aureus and Enterococcus faecalis) and at4 µg/mL against Staphylococcus aureus. Thus, the investigation of antibacterial screening revealed that most of the synthesized compounds were inactive but 6c has shown half antibacterial activity against gram positive while 6d has shown two fold antibacterial activity against Staphylococcus aureus compared to the reference ciprofloxacin. From the results, it is evident that the presence of chloride or trifluoromethyl at 5-position of benzimidazole leads to the appearance of
![]()
Table 2. Melting points and yields of 2-[(1-H-benzimidazol-2-yl)methylthio]-4,5-diphenyl-1H-imidazoles 6a-e.
![]()
Table 3. MIC test results of 4,5-diphenyl-1H-imidazoles derivatives 2a-e and 6a-e.
-No activity.
antibacterial activity.
Overall, the results indicate very useful information for researchers interested in designing antibacterial agents containing imidazole and benzimidazole moities in their structure for both improved potency and for avoiding the chemical space that would not be productive.
4. Conclusion
We have synthesized a series of heterocyclic compounds 2a-g and 6a-e from 4,5-diphenylimidazol-2-thiol 1. The antibacterial activity of compounds 2a-e and 6a-e was evaluated against gram-negative bacteria (Pseudomonas aeruginosa and Escherichia coli) and gram-positive bacteria (Staphyloccocusaureus and Enterococcus faecalis) in a 96-well plate using the liquid dilution method (liquid micromethod) and ciprofloxacin as a reference. Most of the synthesized compounds were inactive but 6c has shown half antibacterial activity against gram-positive while 6d has shown two-fold antibacterial activity against Staphylococcus aureus compared to the reference ciprofloxacin.
Acknowledgements
The authors thank the “Ministère de l’Ensignement Superieur de Côte d’ivoire” for fellowship fund (CB) and the “Laboratoire de Glycochimie, des Antimicrobiens et des Agroressources (LG2A) de l’Université de Picardie Jules Verne, Amiens” for offering access to their instruments and expertise.