Antimicrobial Effectiveness on Selected Bacterial Species and Alkaloid and Saponin Content of Rosa nutkana C. Presl (Nootka Rose) and Urtica dioica L. (Stinging Nettle) Extracts ()
1. Introduction
Indigenous peoples of North America have historically used plants such as Nootka rose (Rosa nutkana C. Presl) and stinging nettle (Urticadioica L.) to heal skin infections by following traditional protocols for plant extract preparation and application. Medicinal plants are a largely unexplored drug repository and some have great potential for novel leads for inhibitors of microbial agents of disease. By the sharing of knowledge of plant medicines by Indigenous Knowledge keepers, Indigenous science has made important contributions to universal health and wellbeing. Statistics continue to show that a large proportion of the world’s population continues to use medicinal plants as preventative maintenance of wellbeing, for their primary health care needs, and/or treatment of disease [1]. In this context, plants are consistently viewed as sources of useful medicines and are considered to have great potential in future applications for improving health, including the healing of infections as highlighted in this study. Recently, plants have been shown to treat infections that are difficult to manage with standard antibiotics, due in part to bacterial species that are becoming increasingly drug-resistant [2] [3]. Plants produce secondary bioactive compounds such as phenols, saponins and alkaloids that are inessential to the normal growth and reproduction of the plant. These compounds found in plant extracts have been analyzed by Western scientific methodologies and have revealed to be protective agents capable of deterring animal consumption and multidrug resistant bacterial species [4] [5]. Because the development of antibiotics resistance is a world-wide health concern, finding new plants to effectively treat infections can be especially helpful and timely.
Nootka rose is native to British Columbia, Canada, and ranges from Alaska to the northwestern states in the USA. Traditional use of the leaves includes application to painful bee stings and abscesses [6]. Studies on the Rosa family have shown the therapeutic potential of its antioxidant activity for the treatment of disorders such as cancer, rheumatoid arthritis, osteoporosis, diabetes, skin disorders, and infectious diseases [7]. Methanolic Nootka rose branch extracts have also exhibited antiviral activity and a high level of effectiveness against an enteric coronavirus [8]. Nootka rose has a significant concentration of total phenolic compounds in its seeds and a markedly higher concentration in its whole hips which show greater antioxidant activity compared to other British Columbia rose species [9]. Extracts of both plant structures positively correlated with antimicrobial activity against Gram-positive bacteria such as Staphylococcus aureus [9] and several methanolic extraction compounds of Nootka rose fruits were also found to be active against methicillin-resistant S. aureus [10].
Stinging nettle is a common plant found throughout North America, Northern Europe, and Asia at large. The genus designation Urtica comes from the Latin verb urere, which means “to burn”, and refers to stinging hairs located on the surface structures of the stinging nettle plant. The stinging sensation of the leaf hairs is caused by several plant chemicals including formic acid, histamine, serotonin, and acetylcholine accompanying impalement of hairs into the skin [11]. In the Cree culture, stinging nettle is used in leaf decoctions to stop hemorrhaging following childbirth, to help reduce the incidence of diarrhea and rid individuals of infection caused by intestinal worms [12]. The plant has also been used as a diuretic, a therapy for joint pain, and as an anti-inflammatory aid for arthritis and rheumatism [13] [14]. Its anti-inflammatory actions in rheumatoid arthritis are attributed to its ability to inhibit the production and release of pro-inflammatory cytokines and T lymphocytes [15]. Stinging nettle shows promising results against inflammation [16], possesses immune-modulatory potential [17] and antimutagenic and radical-scavenging properties that may contribute to chemoprevention of cancers [18]. Water extracts of stinging nettle show antioxidant activity and antibacterial activity [19] and recently these extracts have shown antibacterial activity against multi-drug resistant strains of Mycobacterium tuberculosis [2] and methicillin-resistant Staphylococcus aureus [3].
In addition to antimicrobial activity, secondary bioactive compounds such as alkaloids and saponins also play a role in defending plants against consumption by herbivores [5] [20] [21] [22]. Alkaloids include unpalatable, bitter compounds such as chelerythrine, caffeine, quinine, and scopolamine. Saponins are high molecular weight glycosides that have detergent properties that create stable foams in aqueous solutions [23]: the soapwort plant’s saponin content produces soap-like foam when agitated in water and has been effectively used in traditional liquid soaps [24]. Saponin compounds are usually found in the outer cell layers of plants and provide an effective barrier against establishment of microbial populations. These glycosidic compounds also reduce plant palatability due to the strong bitter flavour, thereby decreasing the frequency of foraging on the host plant [25].
This study is unique due to its partnership between Indigenous Knowledge keepers and researchers: Few prior studies have combined Indigenous science protocols and Western science methodologies. This study examines the effectiveness of traditional Nootka rose and stinging nettle plant preparations, that have been used traditionally by the Indigenous people of British Columbia and Washington to wash the skin [26], against three common bacterial species capable of causing skin infections: Staphylococcus aureus,Micrococcus luteus, and Pseudomonas aeruginosa. The comparative bacterial effectiveness of the plants when prepared according to Indigenous and Western science protocols was considered. Selection of Nootka rose and stinging nettle was based on discussions with Traditional Knowledge keeper S. Johnny of Cowichan Tribes of Vancouver Island in British Columbia, Canada.
2. Materials and Methods
2.1. Plant Material
The fresh above-ground parts of Nootka rose and stinging nettle were collected on Cowichan Tribes land, Duncan, British Columbia, and identified by Traditional Knowledge keeper Stella Johnny. Taxonomic identification of the plant material was also conducted by Dr. Mary Vetter, Luther College, University of Regina, Saskatchewan, and compared with theplantlist.org.
It is important to follow proper protocol when working with Elders and other Traditional Knowledge keepers. Stella Johnny indicated that when each plant is harvested, one must know the area harvested from and “be prepared” to “honour the Gifts given by the Creator [in the form of the harvested plants]”. As the plant is alive, it will be giving up its life for our needs. When one rises the morning of the harvest, it is important to thank the Creator and the plant. The best time to harvest is just before daybreak and a gift of tobacco is offered on the ground where plants are harvested. The Elders shared that when plants are used in research, it is important to be thankful to the plants for the gift of knowledge it represents.
2.2. Soxhlet Extractions and Kirby Bauer Disc Sensitivity Testing
Bacterial species (Staphylococcus aureus (ATCC 25923),Micrococcus luteus (ATCC 4698), and Pseudomonas aeruginosa (ATCC 10145) were cultured in nutrient broth, adjusted to 0.5 McFarland scale and uniformly swabbed onto the surface of nutrient agar plates [27]. Dimethylsulphoxide (DMSO) and methanol were used as negative controls. All procedures were conducted using a class II, Type A2 Biosafety Cabinet (NuAire, Model: NU-540-500).
Plant extract material was obtained by conducting Soxhlet extraction procedures using dried plant material [28], followed by application of a Buchi vacuum controller v-805 rotavapor. Approximately 80 g of dried plant material was used to fill a Soxhlet thimble and 150 ml of methanol (ACS Laboratory grade) was used for the extraction procedure. Final concentration of the extract was done by transferring the above viscous solution into 10 mL, sterilized screw-cap vials and then concentrating the extract directly in the vial under a stream of air.
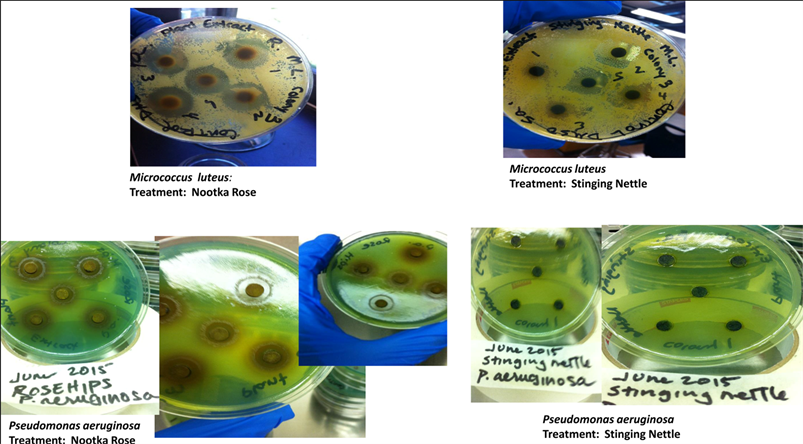
The freshly prepared plant extract solutions (10% sterile DMSO) were filter sterilized using 0.45 µL filter syringes. Sterile filter discs (6 mm) were then saturated with 40 µL of plant extract solution, placed into sterile, covered petri dishes and stored at room temperature in the dark and overnight to remove excess methanol [29]. The filter discs were applied to Mueller Hinton agar plates, following the uniform bacterial swabbing procedures. Plant extracts for each plant species were tested on pure bacterial cultures (× 3 colonies) × five filter discs/agar plate and for each of the three bacterial species studied. The antimicrobial effectiveness for each plant species tested was determined by measuring the zones of inhibition that surrounded each disc (mm). Dimethyl sulfoxide (DMSO) and methanol were used as negative controls.
2.3. Minimum Inhibitory Concentration Values (MIC) and Minimum Bactericidal Concentrations (MBC) Standard Testing with Inclusion of Traditional Knowledge Keeper’s Methods for Plant Medicine Preparation
MIC and MCB testing were employed using the Traditional Knowledge keeper’s direction for picking Nootka rose and stinging nettle plants, hanging/drying the plant and steeping the plant and nutrient broth (30 g/200mL) solution for one hour. Standard methods for MIC and MBC testing were then employed for plant preparations of Nootka rose and stinging nettle at full-strength (30 g/200mL) and following standard dilution procedures. MIC/MBC values are often employed for observing the effectiveness of full strength and serial dilutions of the plant medicine solutions on bacterial growth on agar surfaces [27] [30] [31]. They are performed to determine the minimum concentration for effectiveness of antimicrobial agents and to prevent a toxic application of antimicrobial agent used [32]. Standard methods for determining MIC and MBC include observation and determination of the lowest concentration of tested plant extract that prevents observable bacterial growth [27] [32].
2.4. Bacterial Population Counts
Bacterial population counts (CFU) were conducted using standard procedures [33]. Each plant trial included three S. aureus colonies, three replicates per colony, when using 20 g/200mL plant treatment of Nootka rose and stinging nettle.
2.5. Soxhlet Extraction for Determination of the Plant Biological Compounds
Chemicals used throughout these procedures were of analytical grade (hexanes, HCl, methanol, acetone (from Fisher Scientific, ON, Canada), 95% ethanol and NH4OH (from Sigma-Aldrich, ON, Canada), KOH (from Occidental Chemical Corporation, TX, USA), petroleum ether (from BDH, ON, Canada), and chloroform and dichloromethane (from EMD, ON, Canada). The extraction was performed under ambient conditions at the boiling point of the solvent used.
The obtained plant extracts were cleaned from oily materials by saponifying [28]. The hexane extracts were concentrated under vacuum. The residues were then saponified with 50 mL of 95% ethanol and 2 g of KOH in 50 mL ethanol solution in a hot water bath. The extracts were diluted with 100 ml of distilled water and were shaken with 75 mL and then 2 × 50 mL portions of petroleum ether. The organic phases were collected and the solution obtained was washed with 2 × 50 mL portions of distilled water until neutral pH then evaporated rotary evaporator. The dry extract was weighed and dissolved in approximately 5 mL of CHCl3.
2.6. Determination of Alkaloids by Soxhlet Extraction
Alkaloids were determined following the method of [34] [35]. Two samples of ground rose hips between 10 and 20 grams were extracted with 100 mL of refluxing 100% ethanol (78˚C). Once the ethanol was evaporated, approximately 2 g of dried extract was dissolved in 20 mL of 5% HCl. The mixture was centrifuged for 12 minutes (RT, 6000 RPM) and the aqueous portion was basified with NH4OH solution. The basic solution was extracted three times with CH2Cl2 and concentrated under reduced pressure by rotary evaporator. Once dried, the sample was weighed to determine the amount of alkaloid residue. This process was repeated for two stinging nettle samples between 4 and 14 g.
2.7. Determination of Saponins by Soxhlet Extraction
Saponins were determined following the method of [34] [35]. Two samples of ground rose hips between 10 and 20 grams were extracted with 100 mL of refluxing hexane (69˚C) for 20 minutes. Hexane was separated from the plant extract, which was extracted three times with 30 mL of methanol. The resulting solution was concentrated to one third of its original volume and 100 mL cold acetone was added to this extract. The extract was then filtered by pressure filtration using pre-weighed filter paper (Whatman No. 1 Qualitative Circles 125 mm) and masses of saponins weighed by difference. This process was repeated for two stinging nettle samples between 4 and 14 grams.
2.8. Statistical Analyses
One-way ANOVA and Tukey’s HSD were used to determine statistical differences between the diameters of zones of inhibition using the Kirby Bauer disc diffusion test. Statistical analyses were carried out with the statistical analysis software R (version 3.1.2) and values of p < 0.05 were noted as statistically significant.
3. Results
3.1. Kirby Bauer Disc Sensitivity Testing
Results for zones of inhibition showing equal to or greater than 7 mm (6 mm being the diameter of the filter discs) were considered active against the bacterial populations tested [36]. Nootka rose extracts showed showed significantly greater zones of inhibition (> 12 mm) for all three bacterial species tested when compared to results for stinging nettle extracts, where all zones of inhibition measured at < 12 mm (Table 1). Results for the Nootka rose extracts and M. luteus showed zones of inhibition at 18.6 mm, zones of inhibition for S. aureus measured at 16.77 mm, while P. aeruginosa measured at 12.13 mm. The zones of inhibition with the Nootka rose extracts were also statistically greater than the methanol and DMSO controls which showed no zones of inhibition, measuring at 6 mm. Stinging nettle extracts showed zones of inhibition at 11.6 mm for M. luteus but demonstrated a lack of antibacterial effectiveness against S. aureus andP. aeruginosa. In summary, Nootka rose extracts showed antibacterial effectiveness against all three bacteria while stinging nettle extracts was only effective against M. luteus.
![]()
Table 1. The diameters of inhibition zones (mm) for Staphylococcus aureus, Micrococcus luteus, and Pseudomonas aeruginosa using Nootka rose and stinging nettle plant extracts.
Means within each column with different letters (a)-(c) differ significantly (p < 0.05).
3.2. MIC and MBC Values
When considering results for MIC and MBC, M. luteus showed no growth for Nootka rose for the prepared full-strength plant solution (30 g/100mL), ten-fold or 100-fold serial dilution treatments, while M. luteus growth was observed for all other plant treatment dilutions. For S. aureus no growth was observed for the full-strength plant treatment (except for one colony that may have been a contaminant) or the 10-fold plant treatment dilution, but growth was observed when using the plant treatment diluted by 100-fold. P. aeruginosa showed growth when using the full-strength plant treatment solution and when all other dilutions were tested. Thus, results for Nootka rose plant treatments, developed according to methods using Indigenous science protocol, showed the highest level of effectiveness against M. luteus, followed by S. aureus with the lowest level of effectiveness seen for P. aeruginosa results. When considering stinging nettle, all three bacterial species tested exhibited growth when cultured in full-strength and all other plant treatment dilutions. Given that growth was observed for all plant treatments, the stinging nettle was not effective in growth inhibition for all bacterial species tested.
3.3. S. aureus Bacterial Population Counts
S. aureus population counts (CFU) at 10−6 for the Nootka rose treatment, using 20 g/200mL plant treatment, were at 0 CFU or too few to count (TFTC). Results for the control with no plant extract treatment was too numerous to count (TNTC, > 300 bacterial colonies on the plates). The S. aureus population count at 10−6 for the stinging nettle treatment was similar to the control at TNTC. These results show that when using the advisement of the Traditional Knowledge keeper for plant preparation to conduct bacterial population counts for S. aureus, the Nootka rose treatment shows effectiveness at reducing bacterial populations for S. aureus while stinging nettle showed a lack of effectiveness against S. aureus.
Alkaloid percentage was highest in stinging nettle (0.17%) than Nootka rose (0.07%). On the other hand, saponin percentage was highest in Nootka rose (0.87%) than stinging nettle (0.17%) (Table 2).
![]()
Table 2. Alkaloid and saponin mean percentage (%) in Nootka rose and Stinging Nettle.
4. Discussion
The antibacterial activity of Nootka rose and stinging nettle was considered for both Indigenous science (followed by determination of MIC and MBC results and bacterial population counts) and Western science protocols of plant preparation (tested using Kirby Bauer disc sensitivity testing). When considering both methods of plant preparation, results agreed that Nootka rose was the most effective at inhibiting growth of all bacterial species tested. When Nootka rose plant extract preparation obtained by Soxhlet extraction was used to conduct Kirby Bauer tests, results showed significant antibacterial effectiveness against all bacteria tested with the greatest level of activity against M. luteus, followed by S. aureus, whereas the lowest level of effectiveness was towards P. aeruginosa. When Nootka rose was prepared as advised by the Traditional Knowledge keeper and used for conducting MIC and MBC testing, results were like those obtained when using Soxhlet extraction for the Kirby Bauer method, with the greatest level of effectiveness against M. luteus, followed by S. aureus and the least level of effectiveness against P. aeruginosa. Results showed that growth of M. luteus and S. aureus was inhibited by plant preparations following Indigenous science protocols of the Nootka rose plant, but stinging nettle plant preparations showed a lack of effectiveness.
S. aureus is a Gram-positive bacterium frequently associated with skin infections. Nootka rose sepal extract inhibited the growth of this bacterium in a study looking at patients infected with stye infections [37]. The flavonoid quercetin and hydrolysable tannins found in rose hips have demonstrated antibacterial activity against MRSA [10]. For this study, growth of the Gram-positive S. aureus and M. luteus species was inhibited by Nootka rose plant extracts obtained by Soxhlet extraction and Traditional Knowledge keeper advised Nootka rose plant solutions.
Plant preparations of stinging nettle prepared using the Traditional Knowledge keeper’s advisement showed a lack of effectiveness against S. aureus. This agrees with the results observed when this bacterium was treated with Soxhlet plant extracts during Kirby Bauer disc sensitivity testing. Motamedi et al. [38] found that ethanolic and methanolic extracts of stinging nettle leaves showed antibacterial activity against S. aureus, however the extraction solvent appeared to play an important role toward the outcome for antibacterial susceptibility tests. When examining the antibacterial activity of stinging nettle leaves by the disc sensitivity method, it was found that plant extracts obtained when using the solvent hexane were comparable to standard antibiotic Gentamycin, while chloroform, methanol, ethyl acetate, and aqueous extracts showed moderate to no activity against a variety of bacteria [36].
M. luteus is a Gram-positive bacterium which is commonly found in the oral cavity and the respiratory tract. Stinging nettle showed a high level of effectiveness against M. luteus in the current study, which agrees with a previously conducted study by Gülçin et al. that reported that M. luteus growth was inhibited by water extract of stinging nettle [19]. Stinging nettle’s mode of action against Gram-positive bacteria may include the disruption of the peptidoglycan cell wall layer [38].
In our study, the Nootka rose plant extract obtained by Soxhlet extraction using methanol inhibited the growth of the Gram-negative P. aeruginosa. A lack of effectiveness against P. aeruginosa became apparent when using the Nootka rose plant preparation and Indigenous science protocols for plant preparation to conduct MIC and MBC testing. One study reported that when the methanol extract of Nootka rose sepals was used, the growth of P. aeruginosa was inhibited [37]. Similarly, the rose R. rugosa showed growth inhibitory activity against the Gram-negative bacterium Acinetobacterbaumannii and the phenol antioxidant ellagic acid was identified as the compound shown to possess selective antibacterial activity based on bacteria cell wall differences [9].
Similar to our data, ethanol extracts of stinging nettle leaves showed no antibacterial activity against P. aeruginosa [39]. Motamedi et al. [38] also found that ethanolic and methanolic extracts of stinging nettle leaves were not active against P.aeruginosa [38]. The resistance of Gram-negative bacteria toward stinging nettle extract can be explained by their outer membrane with lipopolysaccharides that acts as a barrier to the entrance of some substances including antibiotics [38].
In this study, Nootka rose showed elevated saponin levels and a greater level of effectiveness against the bacteria tested, when compared with the stinging nettle. In one study with Cyclamen persicum tuber extracts, saponins have shown inhibition of the Gram-positive S. aureus [22]. Saponins extracted from Anabasis articulata (a salt tolerant xerophyte) also showed greater antibacterial activity against both Gram-positive and Gram-negative bacteria compared with alkaloid extracts [40]. Several mechanisms have been proposed to explain the inhibitive effect of saponins on, especially, Gram-positive bacterial growth [21]. It is possible that saponins bind with sterols, causing an increased bacterial membrane permeabilization and thus inhibit bacterial growth by disrupting cell membrane integrity and allowing entry of antibiotic agents into the cytoplasm when used in combination with clinical drugs [21].
A higher percentage for alkaloids was found in the stinging nettle, which showed a lower level of antimicrobial effectiveness compared to Nootka rose. Other studies reported that stinging nettle contains alkaloids, flavonoids, flavonols, lignans, tannins, phenols, and saponins and its antibacterial activity has been attributed to many of these compounds [17] [39] [41] [42]. However, the composition of stinging nettle may differ, potentially caused by differences in planting, climatic, seasonal, and experimental conditions [43]. The extracting solvent may also influence the antibacterial activity of its bioactive components [36]. Other compounds such as terpenes, fatty acid esters and phenols were reported to be the antibacterial compounds in stinging nettle leaves against bacteria [36] [43]. While the previous studies have examined leaves and stems, Kan et al. reported that it was linoleic fatty acid and oleic fatty acid that contributed to its antibacterial activity when S. aureus andP. aeruginosa were exposed to stinging nettle seed oils [44].
It would be helpful to compare the result of this study to those focusing on Nootka rose and stinging nettle from other growth years and locations. More detailed analysis of alkaloid, saponin and antioxidant content via other analytical chemical methods (e.g. UV-vis, HPLC, MS) would also strengthen future literature about this subject.
Antibiotic plant extracts may show value in the form of salves and other extracts, but the authors of this study maintain that the Traditional Knowledge that has made these analyses and discoveries possible should be compensated for in the form of support of Cowichan Tribes of Vancouver Island in British Columbia, Canada.
The current study formed a partnership between Indigenous Knowledge keepers and Western scientists that used methods important to both ways of preparing Nootka rose and stinging nettle for testing against selected species of bacteria known to cause skin infections. Overall conclusions were similar for Nootka rose and stinging nettle when considering the different methods of plant preparation used against the bacterial species selected for this study. Nootka rose showed the greatest level of antimicrobial activity when compared with stinging nettle and was also most effective against M. luteus, followed by S. aureus and least effective against P. aeruginosa for both ways of plant preparation. Nootka rose and stinging nettle plants have historically been used and are presently used to treat skin infections by many Indigenous peoples. Although more research is needed, these medicinal plants can act as promising agents for inhibiting and healing skin infections when working with knowledgeable individuals or be used in concert with newly developed antibiotics at a time when antibiotic resistance is of great concern by the medical community. Heightened interest in the usefulness of plants for treating infections should also come with assurances of appropriate protection of these valuable natural resources.
Acknowledgements
We are grateful to the Elders who shared their Traditional Knowledge with us. This work was supported by grants from the University of Regina President’s Research Seed Grant, First Nations University of Canada Research Awards, the Vancouver Island University Research and Scholarly Activity grant and Social Sciences and Humanities Research Council’s General Research Grant.
Pictures—Supplemental Material