Effectiveness of Using Renalof® in the Elimination of Kidney Stones under 10 mm Located in the Renal-Ureteral Tract ()
1. Introduction
The formation of kidney stones or nephrolithiasis represents a common medical condition, with a worldwide prevalence of more than 10%, which increases annually [1]. Calcium oxalate (OxCa) stones are the most common type of kidney stone. In fact, approximately 80% of all kidney stones contain OxCa as a major component. One of the main issues with nephrolithiasis concerns its high rate of recurrence, which ranges from 35% to 50% after 5 - 10 years and increases to 75% after 20 years onwards. Once an individual suffers from nephrolithiasis the rate of relapse increases and the recurrence interval shortens [2].
Calcium oxalate lithiasis tends to increase with age and begins to form via a crystal produced in the renal tubule cavity, in which it adheres to the surface of renal tubular epithelial cells. In healthy people, most crystals formed in the renal tubule cavity are eliminated in the urine, due to the action of macrophages that dissolve the crystals adhering to the surface of the renal tubular epithelial cells or in lysosomes within the cells. However, in individuals with hyperoxaluria or crystalline urine, renal tubular cells become damaged and, thus, crystallise easily [2] [3].
Urolithiasis is responsible for 1 in 1000 hospitalisations each year. The recurrence rate is high, ranging from 10% to 20% in the first two years and 40% to 60% ten years after the first episode; and is characterised by recurrences and variable morbidity, depending on the region studied. Kidney stones occur at any age but most frequently between 20 and 50 years of age. It presents more frequently in men than in women in a ratio that varies between 2:1 and 3:1, as well as in all ethnicities; and presents in lesser numbers in African-American individuals, but typically in a more advanced form, such as chorale stones [4] [5].
Certain disease presentations have a hereditary factor, such as cystine, uric acid and calcium oxalate stones. Whereas in others, urinary tract infections, climate and the individual’s occupation may constitute a risk factor [5]. Most kidney stones encountered in Latin America are radio-opaque due to their calcium content (calcium oxalate monohydrate, calcium oxalate dihydrate, phosphate and calcium carbonate) [6].
Genetic and environmental factors contribute to the development of kidney stones. During the formation of OxCa crystals, osteopontin (OPN) neutralises the initial nuclei, while increasing the recruitment, migration and adhesion of macrophages and modulating the expression of pro-inflammatory cytokines and interleukins. Its deficiency has been associated with a heightened predisposition to develop such kidney stones [7] [8]. However, the deposition of this OPN-OxCa complex on the renal tubule cell membrane stimulates the enzyme NADPH oxygenase, generating superoxides at the cytosolic level and oxidative damage at the mitochondrial level due to the activation of cyclophilin D and the consequent opening of MPTP [9]. As a consequence of mitochondrial collapse, the apoptosis process is triggered in the renal tubule cells. The crystals lump into a mass and are excreted into the tubular lumen as nuclei of the urinary stones. Finally, kidney stone nuclei containing crystals, cellular debris and stone matrix substances, such as OPN, are produced in tubular lumen (Figure 1).
Clinical diagnosis remains non-specific due to the diverse clinical presentations that may be observed in each patient. Furthermore, a metabolic assessment becomes necessary in order to identify the metabolic and physical/chemical risk factors that may influence the formation of the kidney stone, given the multiple causes that contribute to its occurrence [10] [11].
Though the last two decades have seen considerable improvement in the treatment and prevention of kidney stones via a combination of diet, surgical and pharmaceutical treatments, the side effects of these treatments and the high recurrence rate necessitate alternative strategies for the prevention and treatment of nephrolithiasis. In view of the higher probability of chronic kidney damage due to the repetitive incidence of kidney stone disease, prompt diagnosis and treatment measures are essential to prevent kidney vulnerability.
![]()
Figure 1. Diagram of cell damage in the renal tubule with the presence of calcium oxalate crystals. Neutralisation of OxCa crystals by OPN (1); adhesion of the OPN-OxCa complex to the plasma membrane of the renal tubule cells, increase in ROS (2); initiation of cell apoptosis in cells under ROS and stimulation of inflammation via macrophages (3); formation of OxCa aggregates, OPN and cell debris that lead to kidney stones (4).
The impact that extracorporeal lithotripsy, ureterolithotripsy and percutaneous nephrolithotomy have had on the destruction and elimination of urinary stones, as interventional procedures, have virtually replaced open surgery methods. However, it clearly emerges that the recurrence of kidney stones can be reduced with medical treatment, which selectively targets those patients with recurrent kidney stones, under 10 mm in diameter, supported by therapy not only for physiological, metabolic or physicochemical abnormalities that affect stone formation but also with the use of remedies such as Renalof®, which painlessly and progressively destroys and breaks down stones in the genitourinary system (Figure 2), thereby achieving metabolic inactivity [12] [13].
Kidney damage caused by stones reaches a point of no return since injury to renal epithelial cells promotes the adhesion of OxCa crystals and their retention in the renal tubules, processes which are pivotal in the perpetuation and formation of new kidney stones. In addition to the potential for renal damage from lithotripsy in recurrent patients, prevention provides a new approach [14].
![]()
Figure 2. Diagram of potential mechanism of action of Renalof®. Analgesic and anti-inflammatory effect mediated by salicylic acid (1); diuretic effect and osmotic regulation of mannitol at the level of the nephron (2); control of kidney stone growth by reabsorption of calcium (3); dilution of the stone mediated by magnesium silicate as an ion exchanger (4); elimination of the stone via the urinary tract (5).
For this reason, plants have been used in traditional medicine as a popular treatment for a multitude of pathologies, including kidney stone therapy. Findings drawn from a limited number of scientific studies so far suggest that phytotherapy could serve as an alternative or adjuvant therapy in the treatment of nephrolithiasis [15] [16] [17].
Renalof®, whose main ingredient is an extract of Agropyrum repens enhanced with Molecular Activation Technology (Catalysis S.L., Table 1).
Agropyron repens is a very common species of plant native to Europe. Its rhizome contains numerous roots that extend over a long distance and at a shallow depth, and is considered an invasive species and weed. It has spread to Central Asia and can now also be found in Africa. It is traditionally used as a diuretic and to relieve pain and urinary tract spasms. It also serves as a demulcent and tonic. The plant contains different carbohydrates, mucilaginous substances, pectin, triticin, cyanogenetic glucosides, phenol compounds, flavonoids, soponins, volatile oils, essential oils, vanillin glucoside, salicylic acid, iron and other minerals and large amounts of silica such as magnesium silicate [18] [19].
Among the possible diuretic action mechanisms of Agropyron repens, triticin has been reported to possess antibiotic, urine pH regulating and diuretic properties [20], while mannitol, saponins and flavonoids serve as osmotic regulators at the nephron level [21] - [28], salicylic acid as an analgesic and anti-inflammatory agent [29] [30], and magnesium silicate as an ionic competitor in the formation of calcium oxalate [31] [32] (Figure 1).
Therefore, the purpose of this study was to determine the effectiveness of therapy with Renalof® as an alternative therapy in kidney stones of less than 10 mm in diameter. For that purpose, the main metabolic and physicochemical risk factors were described, as well as the patients’ behaviour according to kidney stone activity pre- and post-treatment. Moreover, the main adverse effects of the treatment were monitored.
Without a doubt, nephrolithiasis is a growing public health problem, often due to insufficient water consumption, exposure to high ambient temperatures or water contamination, without neglecting possible anatomical malformations that can contribute to the development of lithiasis. Faced with this situation, the ideal is to opt for drugs that prevent the evolution of stones that at the time are small in millimetres and that are not obstructive. Renal of is undoubtedly a harmless option, easily accessible, with high expulsion levels, with the presence of scarce adverse reactions which can be managed satisfactorily, obtaining a much higher risk/benefit ratio in favor of the patient.
![]()
Table 1. Qualitative-quantitative composition of the Renalof® capsules product.
Thus, the main endpoint of this study was to determine the time to expulsion or elimination, and secondary endpoints were changes in symptomatology or quality of life associated with renal-ureteral kidney disease and evaluation of study medication adverse events.
2. Materials and Methods
2.1. Product under Study
In this trial, Renalof® (Catalysis S.L., Spain) was the study product used, with a dosage of one 325-mg capsule twice a day before meals (Table 1).
2.2. Study Population
A total of 155 patients were enrolled from August 2019 to July 2020 at the Hospital Universitario Antonio Lenin Fonseca and the Seniors Clinic, in the city of Managua, Nicaragua. Patients were randomly assigned to two groups as per a 4:1 ratio Renalof® treatment group and placebo control group.
Inclusion criteria:
1) Patients over 18 years old, all of whom were mentally and physically able to agree to be part of this study.
2) No existing associated comorbidities or diseases endangering the patient’s stability.
3) No diagnosis of Chronic Kidney Disease, in any stage.
4) Existence of non-obstructive stones under 10 mm located in the renal-ureteral tract.
5) No impairment of renal viability.
Exclusion criteria:
1) Patients under 18 years of age.
2) Existence of associated comorbidities or diseases endangering the patient’s stability.
3) Existence of a diagnosis of Chronic Kidney Disease, in any stage.
4) Occurrence of non-obstructive or obstructive stones over 10 mm located in the renal-ureteral tract.
5) Impairment of renal viability.
6) In the placebo group, patients with a creatinine equal to or greater than 3 ng/dl were excluded to prevent further renal damage.
2.3. Ethics Committee
This study was evaluated and approved by the ethics committee of the Seniors Clinical. Likewise, the patient volunteers gave their consent to enter the study based on the above criteria (Annex I).
2.4. Clinical Trial Design
Randomised, controlled, single-blind, prospective study with two patient groups: 1) Renalof® and 2) placebo, with the same physical characteristics as the experimental product, whereby the patient would not be aware of whether they were receiving one form of treatment or another.
For this purpose, a database was compiled and the descriptive analysis conducted with frequency determination. In all, the study followed 120 patients treated with Renalof® and 35 with a placebo, at a dose of 650 mg BID, for 3 months. A preliminary analysis of the kidney stone types present in the study patients, based on the main compound, was conducted using a biophysical profile via biochemical analysis and the clinical characteristics, medical history and biochemical profile of the creatinine values of the test patients (Table 2). During the trial, systematic monthly imaging tests were performed on all patients by the on-site radiologist and evaluated by the primary researchers. Quality of life tests were performed using specific bimonthly urolithiasis tests to check the efficacy of the treatment (Annex II) and the possible adverse effects of the test product on patients with kidney stones.
![]()
Table 2. Baseline characteristics of study patients enrolled in the trial for the Renalof® and Placebo® groups.
Differences were considered significant for *P < 0.05. aType of metabolic alteration in the event of previous metabolic alterations. bIn the placebo group, patients with creatinine equal to or greater than 3 ng/dl were excluded to prevent further renal damage.
The main variable for this trial was kidney stone clearance with Renalof® treatment in comparison to the placebo, with monthly evaluation during 3 months. Secondary variables included quality of life with established bimonthly measurements and the number of colic episodes during the 3-month follow-up.
2.5. Test Analysis
All patients in the trial were systematically assessed applying the same techniques and procedures. The assessment conducted in the trial comprised ultrasound imaging tests (Philips Clear Vue 650) and initial tomography (16-channel Philips Tomography), and each month during the 4-month follow-up; initial analysis of kidney stone composition by 24-hour clinical urine tests.
Quality of life was established in regard to the presence or absence of symptoms associated with the disease by means of a bimonthly personal questionnaire (Annex II): dysuria or burning on urination, urinary urgency, renal colic, difficulty in carrying out daily activities and periodicity of urinary infections (mild, moderate, severe, yes or no).
Possible adverse effects were evaluated on a bimonthly basis according to the presence or absence of possible adverse effects associated with the product to be evaluated: stomach discomfort, gastric reflux, nausea (mild, moderate, severe, yes or no).
The intensity level of the symptoms referred by the patient was assessed every two months, as well as the level of disability that these could generate. It should be noted that the level of limitation in the patient was minimal, so no dose reduction or suspension of the treatment scheme is warranted.
2.6. Statistics
The baseline characteristics were summarized in number and percentage for categorical variables. The difference of study endpoints between experimental and placebo groups was calculated using Fisher’s exact chi-square test. Endpoints measurements included all patients who were randomized and received at least one dose of study medication (intention-to-treat analysis). We estimated an average of 60% of stone expulsion rate in experimental group. The study was designed to have a statistical power of 95% to detect an absolute difference of 40% in the rates of stone elimination (60% in the group with renalof vs. 20% in the placebo group). Considering a type I error of 0.01 and a type II error of 0.05, 155 patients (120 in Renalof vs 35 in placebo) was needed to reach statistical significance. The level of significance was defined as P ≤ 0.05 (two tailed). All statistical analyses were performed using SPSS software for windows, version 26 (SPSS Inc., USA).,
3. Results
A total of 155 patients were enrolled in the clinical trial. A descriptive baseline analysis (Table 2) was performed comparing the population of the Renalof® group with the placebo group. In the trial group, 65.0% of the patients had been diagnosed with kidney stone disease for the first time, while 80% of the placebo group had experienced recurrent kidney stone disease previously. Most of the stones diagnosed in both groups were calcium-based and smaller than 7 mm in diameter. Likewise, the majority of the two groups did not suffer renal damage (76.60% of the Renalof® group and 97.14% in Placebo) and the most frequent metabolic alteration was hyperuricemia (29.0% and 11.4% respectively).
The results obtained in the study (depicted in Table 3) show a 65% remission of kidney stones in the Renalof® group in the first 8 weeks, compared to only 11.4% elimination in the Placebo group (P < 0.001). Over 12 weeks, until the completion of follow-up, 97.5% of patients in the Renalof® group remitted, compared to 11.4% with persistent stones in the Placebo group (P < 0.001).
Focusing on the symptoms of renal-ureteral kidney stone disease (Table 4), over the 4-month follow-up period, 62.5% of patients in the Renalof® group suffered from associated symptoms such as dysuria, pollakiuria and urinary urgency for 2 weeks, 23.3% for 4 weeks and only 14.2% for 6 weeks or more. However, in the Placebo group, a higher percentage of patients were observed with unaltered persistent symptoms (51.4%) or for 6 or more weeks (42.9%).
With regard to colic (Graph 1), a significant decrease in colic was observed in the Renalof® group during the treatment period, dropping from 62.6%, 29.17% and 8.33% in the first month with 1, 2 or more colic episodes respectively, to 5.83%, 0.83% and 0% after 3 months of treatment. In the placebo group, an
![]()
Table 3. Evolution of the kidney stone clearance period in the study patients.
Differences were considered significant for *P < 0.05.
![]()
Table 4. Variation in the symptomatology associated with renal-ureteral kidney disease in trial patients.
Differences were considered significant for *P < 0.05.
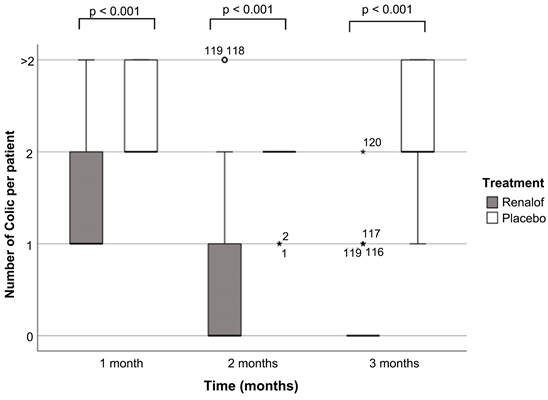
Graph 1. Evolution of the number of colic attacks caused by kidney stones per month of treatment in trial patients in the Renalof® treatment group compared to the control group. Number of colic per patient treated with Renalof (grey) per month; number of colic per patient treated with placebo (white) per month. Differences were considered significant for *P < 0.05.
increase in the number of colic episodes was observed, with most patients suffering at least 2 colic episodes per month (51.43% in the first month; 85.71% in the second month and 42.86% in the third month).
With regard to symptoms and colic, patients with renal-ureteral stones treated with Renalof® experienced an improvement in their quality of life (95.0%) compared to the control group (11.4%) (Table 5).
Finally, only 3.33% of patients in the Renalof® group developed an adverse reaction related to the treatment product (Table 6).
4. Discussion
Kidney stones, especially in terms of small elements, have a high incidence, particularly in tropical countries where they occur frequently owing to the lack of water consumption. The techniques for its treatment approach have changed, initially involving open surgery. Currently, multiple minimally invasive surgery techniques exist, which allow for a rapid recovery of the patient with an excellent rate of lithiasic mass removal.
Prevention of stone formation remains essential, i.e. sufficient water consumption and the correction of metabolic disorders that render patients susceptible to the development of new stones. In this study, most stones presented a calcium aetiology, predominantly in male individuals. The size of the stone was less than 5 mm in 55.83% of cases, without causing renal damage. Most of the patients were managed via ultrasound.
![]()
Table 5. Assessment of the improvement in patients’ quality of life with kidney stones in the Renalof® treatment trial.
Differences were considered significant for *P < 0.05.
![]()
Table 6. Adverse reactions during the treatment.
Differences were considered significant for *P < 0.05.
Results shown in this study corroborate the high rate of effectiveness of Renalof®, within phytotherapy, finding up to 65% clearance in the first 8 weeks of treatment and 97.5% after 12 weeks of treatment in kidney stones of less than 10 mm in diameter.
Likewise, an improvement in the quality of life of these patients was observed, with a reduction in the symptoms associated with renal-ureteral kidney stones such as dysuria, pollakiuria, urinary urgency, as well as a drop in the number of colic episodes throughout the treatment, as shown in the results. This presents a key to controlling such commonplace complications of kidney stones, as well as simplifying lithotripsy surgical treatment itself.
The placebo group had minimal response to the treatment, with poor stone clearance (only 11.4%), with stones being less than 5 mm in diameter. Larger stones did not vary throughout the trial. However, while the size of the stones remained unaltered, the number of colic episodes and associated symptoms rose, especially towards the second half of the trial’s follow-up, a very common occurrence that increases the average cost of this type of treatment.
Minimal adverse drug reactions occurred. Only 3.33% were associated with nausea when using said product, and only possibly due to the rejection of the capsule format. This indicates good product acceptability and safety.
The final profile of the patients treated in the Renalof® group was that of outpatients, in which a prompt remission of the symptoms occurs, which are usually incapacitating and cause the patient to receive medical care. Therefore, the time spent by the patient corresponds to the time spent in the follow-up medical consultation, and the average time spent performing follow-up examinations, such as imaging, or providing a urine sample to the laboratory, without being subject to medical leave or hospitalisation.
For larger stones, a longer treatment time of up to 12 weeks would be required. This would necessitate a study with increased sampling and follow-up time to test the effectiveness of this product in these cases.
For the above reasons, considering that the average time spent in hospital for renal colic averages 3 days, it costs approximately 200 dollars for the use of the bed, food and daily administration of medication. If such a condition is resolved surgically, the length of stay increases to 5 days and the cost rises to 3000 dollars, including the surgical procedure (endourological procedure, with stent placement generally). Furthermore, the average length of medical leave lasts from 15 to 21 days. In the group treated with non-invasive Renalof®, the average cost per patient with renal-ureteral kidney stones would be greatly reduced, diminishing the number of interventions including treatment of kidney stones smaller than 10 mm in diameter and prevention of relapses in recurrent patients.
Therefore, we strongly recommend the inclusion of this product in kidney stone disease management protocols, especially for small-sized kidney stones, where high response and effectiveness have been observed.
5. Conclusion
In this study, the effectiveness of the Renalof® treatment demonstrated a 97.5% effectiveness in the elimination of renal-ureteral stones of less than 10 mm in diameter. Additionally, it improved the associated symptomatology and decreased the number of colic episodes per patient without any noticeable side effects, thereby improving the quality of life and the average cost per patient.
Acknowledgements
We thank Maria Jose Orozco for the permanent support as clinical assistant and for collaborating for the proper follow-up of the patients. We also thank Dr. Melba Isabel Barrantes, for the contribution as a radiologist for the ultrasound follow-up of patients.
Annex I
INFORMED CONSENT
I, hereby declare that Dr. has informed me of the nature of this study, the efficacy of the use of Renalof® in the elimination of kidney stones under 10 mm located in the renal-ureteral tract, as well as the approach to be taken. It was explained that patients will be divided into 2 groups, using either the Renalof® medical product or a placebo at random.
Regardless of the product used, the patient’s evolution will be monitored by means of imaging, both in terms of stone size and quality of life, as well as the reduction of associated symptoms.
Moreover, it was made clear that if the patient wishes to abandon the study for personal reasons, he or she may do so.
Issued in the city of on the of .
Signature: Signature:
Patient: ________________________ Dr. _________________________
Annex II
RATE OF SYMPTOM IMPROVEMENT IN PATIENTS TREATED WITH RENALOF VS. PLACEBO
Answered with mild, moderate, severe, yes or no, as appropriate:
1) Symptoms of dysuria or burning while urinating have decreased:
___________________
2) Presence of urinary urgency:
___________________
3) Presence of renal colic:
___________________
4) Carries out his/her daily activities with normality:
___________________
5) Noticed that the periodicity of urinary infections has decreased:
___________________