Synthesis and Structural Characterization of a New Two-Dimensional Polymeric Hybrid Material {[Cu3(μ3-1,2,3-BTC)(μ2-1,2,3-BTC)(en)3(OH2)2(OH)(μ2-OH2)]2·5H2O·O}n ()
1. Introduction
Polyfunctional hybrid organo-inorganic materials such as metal-organic frameworks (MOFs), microporous organic materials (MOMs) and coordination polymers (CPs) continue to be of great interest for the scientific community owing to their structural and unusual properties as compared with those of activated carbon, silica gel and zeolite [1]. Attractions to this class of materials result not only from the several advantages associated with their intrinsic structural characteristics, but also from their rich physico-chemical properties with potential applications in gas storage, molecular recognition and separation of substrates, and in heterogeneous catalysis [2] [3] [4]. Gas storage is of critical importance in many industrial processes both from an economic and safety point of view. For instance, metal carboxylates such as [Cu2(pzdc)2(pyz)] (where pzdc2− = pyrazine-2,3-dicarboxylate, pyz = Pyrazine) and [Cu3(BTC)2] (BTC3− = benzene-1,3,5- tricarboxylate), characterized by pores radius of 4 × 6 Å and 12 × 12 Å, respectively, are well suitable for acetylene and CO2 adsorption [5] [6]. These abilities of MOFs, MOMs, and CPs to store gases such as methane, ethylene, dihydrogen, carbon dioxide or dinitrogen have led to the replacement of compressed natural gas (CNG) by adsorbed natural gas (ANG), which therefore represents an alternative source of energy in the production of fuels [7].
Continuous research works on materials able to favor gas storage by adsorption are then expected to provide a great contribution in the mitigation of environmental pollution and global warming. In that respect, metal di- or tricarboxylates in association with bidentate amines represent excellent precursors for the generation of coordination polymers that hold potential as inorganic material in the adsorption of small molecules. Some recent examples include the synthesis of 3D-{[Cu(NCCH3)4][Cu3(μ6-btc)2]2(O2CCH3)(H2O,CH3CN)x(btrip)1.5}n from 1,2-bis(1,2,4-triazol-4-yl)propane, in combination with benzene-1,3,5-tricarboxylate [8].
We recently investigated the reaction of several diamine precursors such as 1,3-diaminepropane (tn) with Cu(NDC)·3H2O (NDC2− = naphthalenedicarboxylate) and isolated the complex {[Cu(NDC)(OH2)(μ-OH2)(tn)]n·2H2O} that has been characterized in the solid state by single crystal X-ray diffraction analysis. Its molecular structure revealed a 2D coordination polymer in which the monomeric units are associated by intermolecular hydrogen bonding interactions [9]. In the continuation of this work, we report here the synthesis and structural characterization of a novel CPs based on benzene-1,2,3-tricarboxylatecopper(II) trihydrate (Cu3(1,2,3-BTC)2·3H2O) and ethylenediamine (en) with the former as primary building unit.
2. Experimental
2.1. Materials and Methods
All chemicals were purchased from commercial sources and used as received without further purification. Benzene-1,2,3-tricarboxylatecopper(II) trihydrate was of analytical grade. Elemental analysis was performed using a Fisons Instruments 1108 CHNS-O analyzer. FT-IR spectra were recorded using a Perkin-Elmer FT-IR 100 spectrometer. UV-visible measurements were carried out by the aid of a Jenway 6715 UV-vis spectrophotometer. The melting point of the compound was obtained using an SMP3 Stuart Scientific Melting Point Apparatus while thermogravimetric analysis was carried out using a Perkin-Elmer STA 6000 thermobalance. Magnetic susceptibility was carried out using a Gouy magnetic balance. Reflections were collected on a Bruker SMART APEX II CCD Diffractometer using CuKα (1.54178 Å) radiation at 100 K. Dataprocessing, Lorentz-polarization, and face-indexed numerical absorption corrections were performed using SAINT, APEX, and SADABS computer programs [10] [11] [12]. The structures were solved by direct methods and refined by full-matrix least squares based on F2 with all reflections using the SHELXTL V6.14 program package [13] [14]. Non-hydrogen atoms were refined with anisotropic displacement coefficients. All H atoms were found in electron-density difference maps and carbon-bound H atoms were treated as idealized contribution unless noted differently. Structural data have been deposited with the Cambridge Structure Database as supplementary publications CCDC 1990458.
2.2. Synthesis of {[Cu3(μ3-1,2,3-BTC)(μ2-1,2,3-BTC)(en)3(OH2)2 (OH)(μ2-OH2)]2·5H2O·O}n (1)
A sky blue powder of benzene-1,2,3-tricarboxylatecopper(II) trihydrate (2.0 g, 3.0 mmol) was introduced in methanol (25 mL) and the mixture was stirred for 1h at room temperature. To the blue solution obtained was added ethylenediamine (0.56 g, 9.3 mmol), and the mixture was kept at room temperature under stirring for 24 h, whereby a violet solid precipitated in the reaction flask. The solid was filtered off, washed several times with methanol and dried under air. Recrystallization from methanol by slow partial evaporation of the solvent afforded pure 1 as violet crystals. Yield 2.20 g (72%). Anal. Calcd. (%) for C24H45Cu3N6O21: C 30.53, H 4.80; N, 8.90. Found (%): C 30.34, H 4.97, N 8.76. FT-IR (υ, cm−1): 3496.0 (m), 3400.8 (m), 3107.0 (s), 1595.5 (vs), 1558.9 (vs), 1451.3 (s), 1362.6 (vs), 1280.1 (m), 1157.7 (w), 1122.5 (w), 1052.8 (vs), 927.4 (w), 768.5 (m), 726.2 (w), 704.4 (s) cm−1
3. Results and Discussion
3.1. Synthetic Aspects
Complex 1 was obtained by mixing benzene-1,2,3-tricarboxylatecopper(II) trihydrate, prepared from benwene-1,2,3-tricarboxyates and copper(II) chloride, with excess equivalent of ethylene diamine in methanol at room temperature. The crude product was recrystallized from methanol by slow evaporation and violet crystalline solid of 1 was isolated in good yield (Scheme 1).
Compound 1 displayed a violet color and showed some solubility in organic solvent such as methanol, dimethylformamide and acetonitrile. The material can be handled and stored at air for months without any sign of decomposition.
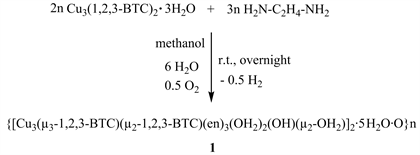
Scheme 1. Synthesis of the two dimensional polymeric complex 1.
3.2. Thermal Properties
TGA (light red curve)/heat flow (dark red curve) profiles of 1 (Figure 1) showed processes with weight loss. The compound showed a 16% weight loss between 40˚C - 120˚C, which can be assigned to the departure of six water molecules (calc. 11.2%) and one molecule of ethylene diamine (6.2%). Above 200˚C, further decomposition of the compound occurs with a final weight loss of 32% at 243˚C and corresponding to the departure of a benzene-1,2,3-tricarboxylic acid molecule (calc. 21.43%) and two molecules of ethylenediamine (calc.12.4%). These decompositions are supported by two endothermic effects at around 97˚C and 210˚C.
3.3. Magnetic Properties
The magnetic properties of the polymeric complex 1 was evaluated by determining the effective magnetic moment μeff of the compound according to the formula μeff = 2.828 × (χMcorT)1/2, where χMcor is the corrected molar susceptibility. Table 1 gives the diamagnetic contribution χa of each atom or group of atoms in 1. The measured value χg was found to be 0.437 × 10−5 and the magnetic susceptibility χM of the compound was calculated to be 0.437 × 10−5 × 1015.38 = 4377 × 10−6.
The corrected magnetic susceptibility is χMcor = (χM − χDia) = 4805 × 10−6 and the effective magnetic moment was deduced to be μeff = 2.828 × (4805 × 10−6 × 292)1/2 = 4.0 BM. This value is close to that of a system with three free electrons (s = 3/2, μ = 3.87 BM) and might suggest a spin-spin coupling that is antiferromagnetic. Ghoshal and coworkers observed antiferromagnetic coupling in the dicarboxylate copper complexes [Cu2(μ-OH2)2(OOC-CH2CH2-COO)(bpy)2(NO3)2]n and [Cu2(μ-OH2)2 (OOC-CH2CH2-COO)(phen)2(NO3)2]n [15].
3.4. IR Spectroscopy
The FT-IR spectrum of 1 (Figure 2) showed that valence vibrations of υas(N-H), υa(N-H), υ(O-H) occur almost in the same region, with a broad and intense absorption band observed at 3496 - 2800 cm−1. The vibration bands of υ(O-H) at 3496 cm−1, expected at about 3500 cm−1, is slightly displaced towards lower frequencies, indicating that the proton is engaged in the hydrogen bonds with oxygen atoms of benzene-1,2,3-tricarboxylate. Similarly, the valence vibration of N-H observed at 3107 cm−1, is slightly above the expected interval 3100 - 2850 cm−1. Similar displacements of N-H stretching frequency due to hydrogen interactions were observed in the reported cadmium [Cd(OAc)(HpzMe, An)(HB{pzAn, Me}3)]·CH2Cl2 and copper compounds [Cu(OAc)(HpzMe, An)(HB{pzAn, Me}3)]·CH2Cl2 [pzMe, An = 3-(p-anisyl)-5-methylpyrazolyl] with pyrazole and acetate as ligands [16] [17] [18]. A lower frequency, adsorption bands at 2850 - 2750 cm−1 are present that were assigned to the C-H valence vibrations of the benzenetricarboxylate ring and of the methyl and ethylenediamine groups [19]. The large and intense absorption band observed between 1595 - 1559 cm−1 is the result of valence vibrations for both C=C and C=O bonds of benzenetricarboxylate. The valence vibration of the C=O group is shifted to lower frequency due to hydrogen bonding interactions between oxygen atoms of the anion and the hydrogen atoms of ethylenediamine or of water. Such a behaviour was also observed in some copper complexes derived from pyridine-2-carboxylate and quinoline-2-carboxylate. The absorption band between 1280 - 1363 cm−1 is due to the stretching and bending vibration of the C=C conjugated bond of the aromatic ring.
T = 292 K, m = 0.104 g, length = 15 mm.
![]()
Table 1. Diamagnetic contributions of atoms or group of atoms in 1.
![]()
Figure 1. Thermal analysis of compound 1. Light red curve (TGA), dark red curve (heat flow), % weight (black line), percent area (red dashed line).
3.5. UV-Vis Spectroscopy
UV-vis spectroscopy analysis of 1 (Figure 3) showed no absorption band in the visible region expected in the range 350 - 800 nm. However, the spectrum shows a single absorption band at 296 nm which is attributed to intraligand π→π* electronic transitions due to C=O, C=C bonds. The broadness of the band and the absorption peak observed is due to the presence of copper that is covalently bound to oxygen.
3.6. X-Ray Single Crystal Structure
Single crystals X-ray diffraction analysis of 1 (Figure 4 and Figure 5, Table 2 and Table 3) showed the formation of a coordination polymer in which the repeating unit is the dimer {[Cu3(μ3-1,2,3-BTC)(μ2-1,2,3-BTC)(en)3(OH2)2(OH)(μ2-OH2)]2·5H2O·O} that is connected by a Cu3-O20 interatomic distance of 2.484(5) Å that is shorter than the van der Waals radii of Cu (2.0 Å) and O (1.55 Å). The extension into a polymer is then assumed by a μ2-bridging benzene tricarboxylates moiety through a stronger interatomic contacts Cu2−O11 of 1.9776(5) Å that is significantly shorter than the Cu3-O20 distance connecting the dimeric unit and slightly longer than the Cu3-O2 (1.958(5) Å) bond length.
The copper atoms in the unit cell exhibit different coordination modes. Indeed, Cu1 is octahedral coordinated by two nitrogen (Cu1-N3/N4 = 1.999(6)/2.009710) Å) and four oxygen atoms from the tricarboxylate unit (Cu1-O4 = 1.956(5) Å) and from three water molecules (Cu1-O14/O18/O20 = 1.983(5)/2.484(5)/2.521(8) Å). In contrast, the Cu2 and Cu3 copper atoms have a square pyramidal coordination geometry formed by two nitrogen (Cu2-N1/N2 = 1.994(6)/1.997(6), Cu3-N5/N6 = 1.985(6)/1.995(5) Å) and three oxygen atoms from the tricarboxylate fragment (Cu2-O2/O11 = 1.958(5)/ 1.9776(5) Å, Cu3-O6/O8 = 1.964(4)/1.958(5) Å), from a hydroxyl group (Cu2-O11 = 2.281(6) Å) and from a water molecule (Cu3-O20 = 2.484(5) Å). While the ethylenediamine is chelating each copper center, the benzene tricarboxylatefragment is triply bonded to three copper atoms in a monodentate manner and establishes a bridge between the repeating unit. The coordination of the 1,2,3-BTC,ethylenediamine and bridging water molecules generates a two-dimensional coordination polymer with 4.33 × 6.68 Å2 quasi-rectangular pores (Figure 6).
![]()
Figure 4. Molecular structure of 1 showing the monomeric unit. The hydrogen and oxygen atoms, and the solvated water molecules were removed for clarity.
![]()
Figure 5. Two-dimensional coordination polymer of 1 showing the interatomic connections.
![]()
Figure 6. Pores observed in the coordination polymer. (left) ball and stick and (right) Spacefill views.
![]()
Table 2. Crystal data and refinement details for {[Cu3(μ3-1,2,3-BTC)(μ2-1,2,3-BTC)(en)3(OH2)2(OH)(μ2-OH2)]2·5H2O·O}n (1).
![]()
![]()
Table 3. Selected bond distances (Å) and angles(˚) for {[Cu3(μ3-1,2,3-BTC)(μ2-1,2,3-BTC)(en)3(OH2)2(OH)(μ2-OH2)]2·5H2O·O}n (1).
4. Conclusion
Violet crystals of the copper complex {[Cu3(μ3-1,2,3-BTC)(μ2-1,2,3-BTC)(en)3(OH2)2(OH)(μ2-OH2)]2·5H2O·O}n were isolated at ambient temperature and analyzed using various techniques. The determination of the molecular structure by X-ray diffraction analysis showed that the material is made up of quasi-rectangular pores capable of capturing small gaseous molecules. The complex is on the other hand characterized by a polymeric nature through Cu-O interatomic interactions. The thermal analysis showed loss of weigh of the compound between 40˚C - 120˚C and above 200˚C, while the magnetic susceptibility indicates that the polymer is either ferromagnetic or antiferromagnetic. Futures work will be dedicated to the application of the polymeric hydrid material in nitrogen sorption.
Acknowledgements
The authors are grateful to Prof Claudio Pettinari of the University of Camerino (Italy) & Prof Álvarez Eleuterio of the University of Seville (Spain) for spectroscopic, Thermogravimetric analyses and X-Ray facilities. A.C.T.K. is grateful to the World Academic of Science (TWAS) for equipment support (under Grant Ref. 18-017 RG/CHE/AF/AC-1-FR3240303619). This work was partially supported by the “allocation spéciale pour la modernisation de la recherche universitaire” from the Ministry of Higher Education (Cameroon).