Analysis of Listeriosis Transmission Dynamics with Optimal Control ()
1. Introduction
Listeriosis is an illness caused by the germ Listeria monocytogenes. Generally, humans are infected with listeriosis after eating contaminated food. Listeriosis mostly affects people with weakened immune systems, pregnant women and newborns. The organism was initially described as a cause of epizootics in veld rodents from South Africa (Tiger River disease) by Pier and in 1926 [1]. The organism remained a laboratory problem until the World War II, when it was officially known to be the cause of neonatal sepsis and meningitis [2]. Listeria monocytogenes is mostly found in the environment and it is responsible for meningoencephalitis and stillbirths in a number of animals. The disease usually occurs in human in the setting of pregnancy, immunosuppressive and as the individual ages [3].
Listeriosis is the major cause of encephalitis in ruminants. This encephalitis is usually referred to as “circling disease” because it occurs in the hindbrain and can lead to ataxia in infected animals before death [2]. Listeriosis can or may be a potential risk for veterinarians who are normally working with infected animals. This revelation from veterinary medicine has made epidemiologists speculate that foodborne infection could be responsible for human Listeriosis [2] [4].
The work done by [5] showed that Listeria monocytogenes is a foodborne pathogen that is responsible for the cause of serious invasive illness, mostly in certain class of individuals including elderly and immune compromised patients, new born children and pregnant women. In a study conducted by [6], Listeria monocytogenes has been rated among the most increasing and major food-associated pathogen and many countries of the European Union have always recorded an annual case of human Listeriosis.
The study conducted by [7] on the incidence and transmission of Listeria monocytogenes in ready-to-eat products in retail and food services environments proved that contamination of food products with Listeria monocytogenes can exist or show up at multiple stages before consumption. A research conducted by [8] showed that Listeria monocytogenes is among the foodborne pathogens responsible for invasive illness in certain class of people. The findings revealed an association between preventive measures and reduction on human Listeriosis. Listeriosis is an invasive illness that mainly attacks immune compromised individuals, neonates and pregnant women. The commonest sources of getting infected with the disease are raw milk and meats [9] [10].
A study conducted by [11], revealed that Listeriosis in human are rare but it is among the top serious foodborne diseases in susceptible and vulnerable individuals in a population such as the immune compromised and pregnant women. The resurgence of foodborne Listeriosis was investigated by [12]. The significance of separating the pathogens as a necessary requirement for a correct epidemiological research and eradicating transmission cannot be overemphasised. Moreover, [13], stated Listeriosis as one of leading causes of death from foodborne pathogens. The disease continues to spread and cause sporadic outbreaks of illness.
Research on the epidemics of Listeriosis revealed that transmission of Listeria monocytogenes in food is responsible for human diseases in the early 1980s [3]. An investigation conducted by [14] showed that Listeria monocytogenes are the causative agent of gastrointestinal. The intestinal tract can be the main point of entry for Listeria monocytogenes. [15] conducted a research on the identification and reservoir of pathogens for effective control of sporadic disease and epidemics. The dairy farm has been observed as a potential point and reservoir for Listeria monocytogenes.
In recent times, mathematical models describing the phenomenon and dynamics of infectious diseases have played a key role in the control of diseases in epidemiology. Some of the models are able to explain the dynamics and mode of disease transmission [16] [17]. Complex transmission dynamics of some diseases such as periodic orbits, Hopf bifurcations and multiple equilibria have been described [10] [18].
This is a situational report on Listeriosis outbreaks in South Africa. The data in Table 1 gives the outcome of persons with laboratory-confirmed Listeriosis by province in South Africa. The names of these provinces have been abbreviated for convenience purposes. The data is from the National Institute for Communicable Diseases (NICB), a division of the National Health Laboratory Services (NHLS). This data is between January, 2017 and March, 2018.
![]()
Table 1. Outcome of 967 persons with laboratory-confirmed Listeriosis by province in South Africa between January, 2017 and March, 2018.
Figure 1 shows the situational report of dead and discharged individuals affected with Listeriosis in South Africa between January, 2017 and March, 2018.
![]()
Figure 1. Listeriosis in 9 provinces of South Africa between Jan., 2017 and March, 2018.
2. Listeriosis Model Description and Formulation
The Listeriosis model is divided into two parts, human and vector populations as shown in Figure 2. The populations at any time t are also divided into six subcompartments with respect to their disease status in the system. Human population represented by (
), is divided into subpopulations of Susceptible humans (
), Infected humans (
), and Recovered humans (
).
The total human population is given by:
(1)
The animal population represented by
, is divided into subpopulations of Susceptible animals (
), Infectious animals (
), and Recovered animals (
).
The total vector population becomes:
(2)
![]()
Figure 2. Flow diagram of Listeriosis model.
Table 2 shows the Listeriosis model variables and their interpretations as used in the model formulation.
![]()
Table 2. Listeriosis model variables and their interpretations.
Table 3 shows Listeriosis model parameters with their interpretations as used in model formulation.
![]()
Table 3. Listeriosis model parameters and their interpretations.
Humans can be infected with Listeriosis through ingestion of contaminated foods from infected animals, inhalation of spores and contact with infected animals and humans at a rate
. Listeriosis can be acquired through contacts with infected animals and humans at a rate
, where,
.
The following system of ordinary differential equations is obtained from the model:
(3)
3. Listeriosis Model Analysis
3.1. Positivity and Boundedness of Solutions
The Listeriosis model is epidemically meaningful if all the solutions with non-negative initial data remain non-negative at every point in time.
Theorem 1 Let
then the solution of
is non-negative at all time
.
This implies that, if
are non-negative, then
are also non-negative for all time
.
Human population at any time, t is given by:
(4)
(5)
(6)
In the absence of mortality due to Listeriosis infections:
(7)
As
, the population size,
.
and
.
Also, if
, then
.
(8)
Vector (livestock) population at any time (t) is given by:
(9)
(10)
(11)
In the absence of mortality due to Listeriosis infections:
(12)
As
, the population size,
.
and
.
Also, if
, then
.
Therefore,
(13)
Feasible region is given by:
(14)
where,
(15)
and
(16)
where
is positively invariant.
3.2. Disease-Free Equilibrium
By setting the system of equations in (3) to zero, we obtain the DFE of the model. However, DFE, there are no infections. Hence;
and
The DFE of the Listeriosis model is given by;
(17)
3.3. Listeriosis Reproductive Number
Using the “Next Generation Matrix” approach, we determine
and its linear stability. Listeriosis reproductive number refers to the number of secondary cases produced on average by one infected animal or person in a completely susceptible population. This combines the biology of infections with the social and behavioural factors influencing contact rate [19] [20] [21]. It is the threshold parameter that determines or governs the spread of a disease.
Considering only the infection classes in the system in (3):
(18)
Let f be the number of new infection coming into the system and v be the number of infections that are leaving the system either by death or birth.
,
.
The Jacobian matrix of f and v at disease free equilibrium is obtained by F and V as follows:
(19)
(20)
(21)
(22)
Now, computing the eigenvalues of
and selecting the dominant eigenvalue. Let A represent the eigenvalue of the matrix.
(23)
(24)
and
.
Dominant eigenvalue is
. This implies that;
(25)
Hence,
is given by;
(26)
where:
, for human population.
, for animal population.
3.4. Local Stability of the Disease Free Equilibrium
Theorem 2. The disease free equilibrium is locally asymptotically stable if
and unstable if
.
The DFE was obtained as;
.
The Jacobian matrix of the system is given by:
(27)
where:
Jacobian matrix at disease free equilibrium:
(28)
Determining the eigenvalues:
(29)
The eigenvalues are as follows:
,
All eigenvalues are negative. This implies that disease free equilibrium is locally asymptotically stable [22] [23] [24].
3.5. Global Stability of the Disease-Free Equilibrium
Theorem 3. If
, the disease-free equilibrium is globally asymptotically stable in the interior of
.
Proof: By considering the Lyapunov function:
(30)
Computing the time derivative of P along the solutions of the system in (3);
(31)
The time derivative of P along the solutions of the system of differential equations in (3):
, if and only if
, if and only if
or
.
Highest compact invariant set in
, if
is the singleton
.
Hence,
globally asymptotically stable in
, by LaSalle’s invariant principle [10] [25].
3.6. Endemic Equilibrium
Consider the system in (3), at equilibrium,
This corresponds to the DFE or the relation:
(32)
Let
where:
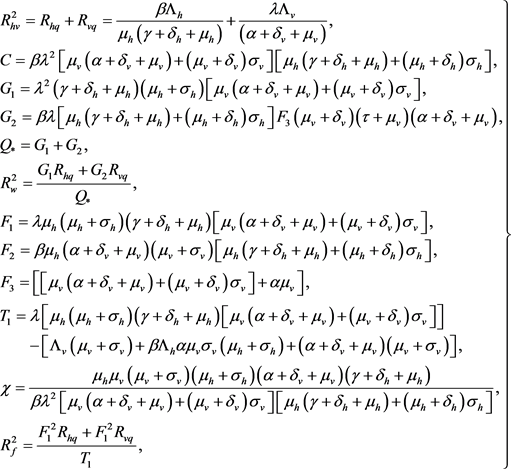 (33)
(33)
![]()
Table 4. Possible positive real roots of
for
and
.
Remark. The system in Equation (3) is said to have an EE, (
), if
. This is satisfied by cases (2, 4, 6) as shown in Table 4. The system in (3) can have more than one EE point if
. This is satisfied by case (8) as shown in Table 4. The system in (3), has more than one equilibrium point if
, as satisfied by case (3, 5, 7).
3.7. Global Stability of Endemic Equilibrium
In this section, the global behaviour of the system in (3) is analysed.
Theorem 4. The system of differential equations in Equation (3), is said to have a unique endemic equilibrium if
, and it is globally asymptotically stable.
The EE can only exist if and only if
. So by letting
, it implies that the EE exists.
Considering the Lyapunov function defined by:
(34)
Computing the derivative of L along the solution of the system in (3) directly;
(35)
Hence:
(36)
This can also be written as:
(37)
Given:
(38)
where M and N are positive and negative respectively.
Therefore:
(39)
and
(40)
Imposing the condition that if
, then the derivative of the Lyapunov function with respect to time is less than or equal to zero.
If
, then
.
But
, if and only if:
The largest invariant set in:
(41)
is singleton
, where
is the EE.
Since all the model parameters are assumed to be non-negative, then the derivative of the Lyapunov function is less than or equal to one, if
of the system in (3) is greater than one, (
). Hence by LaSalle’s Invariant Principle, as t approaches infinity, all the solution of the system in (3) approaches the EE point if
, [25] [26]. Hence, EE is globally asymptotically stable in the invariant set if
.
4. Sensitivity Analysis of Rhv
In this section, we determine the robustness of a model to parameter values. This concept identifies parameters with high impact on
. Using the approach in [20] [27] [28], we determine the sensitivity indices of
, with respect to parameter values in the model.
In biological models, the value of
determines the tendency of the disease to spread within the population. Considering all the parameters of
, we compute the sensitivity of
to each parameter and are represented in Table 5. A positive sensitivity index implies that the parameter contributes to the rise in the basic reproduction number.
![]()
Table 5. Sensitivity indices of parameters to
.
5. Listeriosis Model Extension to Optimal Control
In this section, we carried out an analysis of optimal control to determine the impact of all intervention of the control schemes. This is derived by incorporating the following controls into the model in (3) and the introduction of an objective functional that seeks to minimise:
, where
denotes prevention of
.
denotes treatment of
and
denotes treatment of
.
By introducing all the controls, the system in model (3) becomes:
(42)
In epidemiological models, the essence of optimal control analysis is to minimise the spread or number of infections and the cost of treatment, preventive measures and vaccination controls. The objective functional that can be used to achieve this is given by:
(43)
subject to the system of differential equations in (3).
Where;
are weight constants to aid balance terms in integral so as to avoid the dominance of one another.
, are costs associated with
and
respectively.
, is cost associated with prevention of
.
, is costs of treatment of
and
, is cost associated with treatment of
. Where,
, is period of intervention. Hence,
denotes a linear function for cost associated with infections and
, denotes a quadratic function for the cost associated with controls [29] [30] [31].
Control efforts of model in (3) are by linear combination of
,
. It is assumed to be a quadratic in nature by the assumption that cost is generally non-linear in nature. Thus, the aim is to minimise the number of infection and reduce cost of treatment. The objective is to find the optimal functions
such that;
(44)
where:
denotes the control set.
Pontryagin’s Maximum Principle
This principle provides the necessary conditions that an optimal must satisfy. It changes the system in (3) and Equation (43) into minimisation problem point-wise Hamiltonian (H), with respect to
.
(45)
where:
are referred to as the adjoint variables.
The adjoint (co-state) variables are solutions of adjoint systems below:
(46)
This satisfies the transversality condition:
(47)
By combining the Pontryagin’s Maximum Principle and the existence of the optimal control [32] [33] [34].
Theorem 5. The optimal control vector
that maximises the objective function (J) over
, given by:
(48)
where:
are the solutions of Equation (46) and (47).
Proof. The existence of an optimal control is as a result the convexity of the integral of J with respect to
and
, the Lipschitz property of the state system with respect to the state variables and a priori Boundedness of the state solutions [33]. The system in (46) was obtained by differentiating the Hamiltonian function and evaluated at optimal control. However, by equating the derivatives of the Hamiltonian with respect to the controls to zero, the following are obtained:
In conclusion, by standard control arguments involving bounds on controls:
(49)
The system in (48) leads to system in (47) in Theorem (5). The optimal control uniqueness for small
was gotten as a result of the Lipschitz structure of system of equations and the priori boundedness of the state solutions and adjoint functions. Existence of optimal control uniqueness is in line with uniqueness of optimal system, that comprises of Equations (3), (46), (47) and (48) [20] [22].
6. Numerical Results
In this section, the optimal system was solved using Range-Kutta fourth order scheme. This was done by solving the state systems, adjoints equations and the transversality conditions.
This is a two point boundary value problem and it has two separate boundary conditions at times
and
. The objective is to solve for the value,
days. This was chosen on the basis of the assumption that a period of four month is enough for the disease spread.
Numerical simulation was done by solving the state equations of the model in Figure 2 using Range-Kutta fourth order scheme by making a guess on controls over a simulated time.
Secondly, the use of current iteration of state equations of Figure 2, the adjoint equations and the transversality conditions by a backward method. These controls are then updated by use of convex combination of controls in the previous iterations and values from characterizations of the system.
This process is repeated and iteration is stopped when values of unknowns at the previous iteration are close to those at present iteration [35].
The following combinations of optimal control were considered and the best three most effective selected: Treatment and prevention of humans, treatment of animals and prevention of humans, treatment of animals and humans, prevention of humans and treatment of animals, treatment and prevention of humans, Prevention of humans only, treatment animals only and treatment humans only.
Table 6 shows values of parameters and variables used in the simulation of the model in Figure 2. Some of these parameters were assumed and others taken from published data.
![]()
Table 6. Variable and parameter values of Anthrax model.
6.1. Strategy 1: Optimal Prevention of Humans and Treatment of Animals
Objective functional is optimised by using the prevention control on humans, (
) and the treatment control on animals (vector), (
) by setting treatment control on humans to zero. Figure 3 shows significant reduction in the number of infected animals (vectors), (
) and infected humans, (
). Figure 4 shows the dynamics of recovery. The epidemiological implication; the spread of Listeriosis can be tackled effectively through treatment of animals and prevention of humans. Hence, there should be effective prevention of humans as well as treatment of animals (vectors) in the system.
![]()
Figure 3. Simulation of Listeriosis model: optimal prevention of humans and treatment of animals.
![]()
Figure 4. Simulation of Listeriosis model: optimal treatment of animals and prevention of humans.
6.2. Strategy 2: Optimal Prevention of Humans and Treatment of Humans
The treatment control, (
) of infected humans and prevention of humans, (
) were used in optimising the objective functional and setting (
) to zero. Figure 5 shows that this strategy has caused a substantial reduction in the number of infected animals, (
) and infected humans, (
) drastically. Figure 6 showed the dynamics of the recovered population. In order to achieve this optimal strategy, there should be more treatment of infected humans and prevention of susceptible humans.
![]()
Figure 5. Optimal treatment of infected humans and prevention of susceptible humans.
![]()
Figure 6. Optimal treatment of infected humans and prevention of susceptible humans.
6.3. Strategy 3: Optimal Treatment of Animals and Treatment of Humans
The objective functional was optimised by using (
) and (
) and setting the prevention control on humans, (
) to zero. This strategy resulted in reduction of both infected animals, (
) and humans, (
) as indicated in Figure 7. Biological implication; Listeriosis can be controlled by frequent treatment of animals and humans simultaneously. Moreover, Figure 8 shows the dynamics of the recovered populations.
![]()
Figure 7. Simulation of model: optimal treatment of infected animals and treatment of humans.
![]()
Figure 8. Simulation of model: optimal treatment of animals and treatment of humans.
7. Analysis of Cost Effectiveness
In this section, we conduct an analysis of cost effectiveness to justify the benefits associated with treatment of humans, prevention of humans and treatment of animals. We consider infection averted ratio (IAR) approach and incremental cost effectiveness ratio (ICER) approach.
7.1. Intervention Averted Ratio (IAR)
This is the ratio of number of infection averted to the number of recovered. Infection averted refers to the difference in infected population without control to infected population with control. The ratio for each strategy was computed by using the parameter values of the model. Strategy with highest ratio is considered the most effective intervention to be implemented.
(50)
Table 7 reveals (IAR) for each intervention strategy. Strategy 3 has highest ratio as shown in Table 7 and Figure 9 and therefore considered the best intervention to be implemented in combating the infection in the system. Strategy 2 has the second highest ratio and should be a priority in a situation where two alternative strategies were to be considered. We can conclude that treatment of infected humans and animals should be a priority intervention in fighting the spread of Listeriosis infection. This can be achieved by educating farmers on the need to report any sign of Listeriosis infection for treatment. Health authorities should as well educate the public on the need to treat any suspected Listeriosis infection. Moreover, since prevention and treatment control on humans has the second highest ratio, there should be campaign on prevention and treatment of Listeriosis on humans.
![]()
Figure 9. A plot of strategies against infection averted ratio.
7.2. Incremental Cost Effectiveness Ratio (ICER)
It can be both labour and cost intensive to eradicate a disease in an environment. It is therefore important to consider the best intervention strategy to be implemented. This calls for cost effectiveness analysis to determine the most intervention approach to be used. We can obtain this by comparing the various costs and benefits of these interventions. The analysis of ICER assumes that the costs of the various interventions are proportional to the number of controls employed. Competing strategies are usually compared incrementally by comparing one strategy to the next less effective alternative. The total cost function for each strategy is computed from the various costs functions.
Generally, ICER is applied by first arranging all strategies in increasing order of infection averted as shown in Table 8.
![]()
Table 8. Total infected averted and total cost.
ICER is implemented by simulating the model with all the interventions or strategies. The control strategies are then ranked in increasing order of effectiveness depending on infections averted.
Considering strategy A as the base line and comparing ICER (1) and ICER (3);
and
Comparing the ICER for 1 and 3, we reject intervention strategy 1 since it is most expensive to implement as compared to strategy 3. It saves 3.5070 more than strategy 1.
Now comparing strategy 3 and 2 by computing the ICER (3) and ICER (2);
and
Comparing ICER (2) and ICER (3), we reject intervention strategy 3 since it is most expensive to implement as compared to strategy 2. It saves 7.5014 more than strategy 3. In conclusion, the most expensive intervention strategy to implement is the prevention of humans and treatment of infected animals, strategy 1. However, the most effective and less cost strategy to be implemented is the prevention of susceptible humans and treatment of infected humans, strategy 2.
8. Conclusions
It was established that the model exhibited existence of multiple endemic equilibria. The epidemiological implications are that, effective control of Listeriosis can be achieved if
, is less than unity.
We then carried out the sensitivity analysis of the basic reproduction number, (
). This analysis showed that, increasing livestock recovery rate, would cause a decrease in the basic reproduction number, (
). Moreover, decreasing livestock recovery rate, would increase the basic reproductive number, (
). Also, increasing human transmission rate and livestock transmission rate, would cause an increase in the basic reproduction number, (
) and decreasing human transmission rate and livestock transmission rate, would cause a corresponding decrease in the basic reproduction number,
.
The rate of Listeriosis infection can be reduced by ensuring that the rate of interaction between susceptible humans and infected animals, (β) is minimised. Moreover, the spread of Listeriosis infection can be curbed by reducing the rate of interaction between susceptible animals and contact with infected animals.
The qualitative analysis of optimal control was performed and the necessary conditions for the optimality of Listeriosis disease were analysed. The three most effective strategies according to our model are as follows: the combination of treatment of infected vectors, (
) and treatment of infected humans, (
), the combination of prevention of susceptible humans, (
) and the treatment of infected animals, (
) and the combination of prevention of susceptible humans, (
) and the treatment of infected humans, (
).
The best and most effective intervention approach is strategy 3 as shown in Table 7. Strategy 2 has the second highest ratio and should be a priority in a situation where two alternative strategies were to be considered. We can conclude that the treatment of infected humans and animals should be a priority intervention in fighting the spread of Listeriosis infection. Alternatively, by implementing the incremental cost effectiveness ratio, the best intervention strategy to be considered is strategy 2.
Acknowledgements
We sincerely acknowledge the support of faculty members for their words of encouragement. Our heartfelt appreciation goes to the numerous review comments and suggestions. We express our profound gratitude to the National Institute for Communicable Diseases (NICB), a division of the National Health Laboratory Services (NHLS), South Africa for data on Listeriosis.
Source of Funding
There are no sources of funding for this research. Authors are solely responsible for the entire cost of this research.
Data Availability Statement
The data supporting this compartmental model analysis are from National Institute for Communicable Diseases (NICB), a division of the National Health Laboratory Services (NHLS), South Africa. Some of the parameter values are assumed and others are taken from published articles and are cited in Table 3 of this paper. These published articles are also cited at relevant places within the text as references.
Conflict of Interest
We declare that there is no conflict of interest regarding the publication of this paper.
References
- 1. Murray, E.G.D., Webb, R.A. and Swann, M.B.R. (1926) A Disease of Rabbits Characterised by a Large Mononuclear Leucocytosis, Caused by a Hitherto Undescribed Bacillus Bacterium Monocytogenes (n. sp.). The Journal of Pathology and Bacteriology, 29, 407-439. https://doi.org/10.1002/path.1700290409
- 2. Schlech, W.F. and Acheson, D. (2000) Foodborne Listeriosis. Clinical Infectious Diseases, 31, 770-775. https://doi.org/10.1086/314008
- 3. Schuchat, A., Swaminathan, B. and Broome, C.V. (1991) Epidemiology of Human Listeriosis. Clinical Microbiology Reviews, 4, 169-183. https://doi.org/10.1128/CMR.4.2.169-183.1991
- 4. Osman, S. (2019) Mathematical Modelling of Anthrax and Listeriosisco-Dynamics with Optimal Control. PhD Thesis, PAUST, JKUAT.
- 5. Rebagliati, V., Philippi, R., Rossi, M., Troncoso, A., et al. (2009) Prevention of Foodborne Listeriosis. Indian Journal of Pathology and Microbiology, 52, 145. https://doi.org/10.4103/0377-4929.48903
- 6. Jemmi, T. and Stephan, R. (2006) Listeria Monocytogenes: Food-Borne Pathogen and Hygiene Indicator. Revue Scientifique et Technique, 25, 571-580. https://doi.org/10.20506/rst.25.2.1681
- 7. Lianou, A. and Sofos, J.N. (2007) A Review of the Incidence and Transmission of Listeria Monocytogenes in Ready-to-Eat Products in Retail and Food Service Environments. Journal of Food Protection, 70, 2172-2198. https://doi.org/10.4315/0362-028X-70.9.2172
- 8. Rossi, M.L., Paiva, A., Tornese, M., Chianelli, S. and Troncoso, A. (2008) Listeria Monocytogenes Outbreaks: A Review of the Routes That Favor Bacterial Presence. Revista chilena de infectologia: Organo oficial de la Sociedad Chilena de Infectologia, 25, 328-335. https://doi.org/10.4067/S0716-10182008000500002
- 9. Swaminathan, B. and Gerner-Smidt, P. (2007) The Epidemiology of Human Listeriosis. Microbes and Infection, 9, 1236-1243. https://doi.org/10.1016/j.micinf.2007.05.011
- 10. Osman, S. and Makinde, O.D. (2018) A Mathematical Model for Co-Infection of Listeriosis and Anthrax Diseases. International Journal of Mathematics and Mathematical Sciences, 2018, Article ID: 1725671. https://doi.org/10.1155/2018/1725671
- 11. Little, C.L., Pires, S.M., Gillespie, I.A., Grant, K. and Nichols, G.L. (2010) Attribution of Human Listeria Monocytogenes Infections in England and Wales to Ready-to-Eat Food Sources Placed on the Market: Adaptation of the Hald Salmonella Source Attribution Model. Foodborne Pathogens and Disease, 7, 749-756. https://doi.org/10.1089/fpd.2009.0439
- 12. Allerberger, F. and Wagner, M. (2010) Listeriosis: A Resurgent Foodborne Infection. Clinical Microbiology and Infection, 16, 16-23. https://doi.org/10.1111/j.1469-0691.2009.03109.x
- 13. Donnelly, C.W. (2001) Listeria Monocytogenes: A Continuing Challenge. Nutrition Reviews, 59, 183-194. https://doi.org/10.1111/j.1753-4887.2001.tb07011.x
- 14. Hof, H. (2001) Listeria Monocytogenes: A Causative Agent of Gastroenteritis? European Journal of Clinical Microbiology and Infectious Diseases, 20, 369-373. https://doi.org/10.1007/PL00011277
- 15. Borucki, M.K., Reynolds, J., Gay, C.C., McElwain, K.L., Kim, S.H., Knowles, D.P. and Hu, J.X. (2004) Dairy Farm Reservoir of Listeria Monocytogenes Sporadic and Epidemic Strains. Journal of Food Protection, 67, 2496-2499. https://doi.org/10.4315/0362-028X-67.11.2496
- 16. Karanja, T.W., Osman, S. and Wainaina, M. (2019) Analysis and Modelling of Ringworm Infections in an Environment. Global Journal of Pure and Applied Mathematics, 15, 649-665.
- 17. Osman, S., Musyoki, E.A. and Ndungu, R.M. (2019) A Mathematical Model for the Transmission of Measles with Passive Immunity. International Journal of Research in Mathematical and Statistical Sciences, 6, 1-8. https://doi.org/10.9734/arjom/2019/v12i330090
- 18. Eyaran, W.E., Osman, S. and Wainaina, M. (2019) Modelling and Analysis of Seir with Delay Differential Equation. Global Journal of Pure and Applied Mathematics, 15, 365-382.
- 19. Muia, D.W., Osman, S. and Wainaina, M. (2018) Modelling and Analysis of Trypanosomiasis Transmission Mechanism. Global Journal of Pure and Applied Mathematics, 14, 1311-1331.
- 20. Makinde, O.D. and Okosun, K.O. (2011) Impact of Chemo-Therapy on Optimal Control of Malaria Disease with Infected Immigrants. BioSystems, 104, 32-41. https://doi.org/10.1016/j.biosystems.2010.12.010
- 21. Nyasagare, B.N., Osman, S. and Wainaina, M. (2019) Modelling and Analysis of Campylobacteriosis in Human and Animal Populations. Global Journal of Pure and Applied Mathematics, 15, 551-567.
- 22. Makinde, O.D. (2007) Adomian Decomposition Approach to a Sir Epidemic Model with Constant Vaccination Strategy. Applied Mathematics and Computation, 184, 842-848. https://doi.org/10.1016/j.amc.2006.06.074
- 23. Eustace, K.A., Osman, S. and Wainaina, M. (2018) Mathematical Modelling and Analysis of the Dynamics of Cholera. Global Journal of Pure and Applied Mathematics, 14, 1259-1275.
- 24. Kanyaa, J.K., Osman, S. and Wainaina, M. (2018) Mathematical Modelling of Substance Abuse by Commercial Drivers. Global Journal of Pure and Applied Mathematics, 14, 1149-1165.
- 25. LaSalle, J.P. (1976) The Stability of Dynamical Systems, Regional Conference Series in Applied Mathematics. SIAM, Philadelphia.
- 26. Osman, S., Makinde, O.D. and Theuri, D.M. (2018) Mathematical Modelling of Transmission Dynamics of Anthrax in Human and Animal Population. Mathematical Theory and Modelling, 8, 47.
- 27. Karunditu, J.W., Kimathi, G. and Osman, S. (2019) Mathematical Modeling of Typhoid Fever Disease Incorporating Unprotected Humans in the Spread Dynamics. Journal of Advances in Mathematics and Computer Science, 32, 1-11. https://doi.org/10.9734/jamcs/2019/v32i330144
- 28. Osman, S, Makinde, O.D. and Theuri, D.M. (2018) Mathematical Modelling of Anthrax with Optimal Control. JKUAT Annual Scientific Conference Proceedings, 54-72.
- 29. Joshi, H.R., Lenhart, S., Li, M.Y. and Wang, L.C. (2006) Optimal Control Methods Applied to Disease Models. Contemporary Mathematics, 410, 187-208. https://doi.org/10.1090/conm/410/07728
- 30. Okosun, K.O. and Makinde, O.D. (2014) A Co-Infection Model of Malaria and Cholera Diseases with Optimal Control. Mathematical Biosciences, 258, 19-32. https://doi.org/10.1016/j.mbs.2014.09.008
- 31. Osman, S., Makinde, O.D. and Theuri, D.M. (2018) Stability Analysis and Modelling of Listeriosis Dynamics in Human and Animal Populations. Global Journal of Pure and Applied Mathematics, 14, 115-137. https://doi.org/10.1155/2018/1725671
- 32. Pontryagin, L.S. (1987) Mathematical Theory of Optimal Processes. CRC Press, Boca Raton.
- 33. Fleming, W.H. and Rishel, R.W. (2012) Deterministic and Stochastic Optimal Control, Volume 1. Springer Science & Business Media, Berlin.
- 34. Osman, S., Otoo, D., Makinde, O.D., et al. (2020) Modeling Anthrax with Optimal Control and Cost Effectiveness Analysis. Applied Mathematics, 11, 255. https://doi.org/10.4236/am.2020.113020
- 35. Van den Driessche, P. and Watmough, J. (2002) Reproduction Numbers and Sub-Threshold Endemic Equilibria for Compartmental Models of Disease Transmission. Mathematical Biosciences, 180, 29-48. https://doi.org/10.1016/S0025-5564(02)00108-6