1. Introduction
Chlorhexidine (CLX) is the gold standard mouthwash used as an adjunctive treatment for oral health [1]. However, studies have shown that CLX has a cytotoxic effect on numerous cells such as cells of the periodontal ligament 2 and osteoblasts [2], besides inhibiting collagen and protein synthesis [3] [4] [5] [6]. It has also been shown to reduce the adhesion of gingival fibroblasts to fibronectin and prevent fixation to the root surface, affecting healing [2]. It is important to consider that the stratified squamous epithelium of the gingiva is mainly composed of keratinocytes and is responsible for creating and maintaining a barrier for the oral mucosa [7] and the cytotoxic effects of CLX on these cells have also been demonstrated, which may impair the healing processes [8]. These findings show a trend towards the establishment of a differentiated mouthwash protocol for post-control immediate operative period, thus limiting the use of CLX.
Pakdaman presented Oral Oxygen Therapy in Germany, with promising prospects for the treatment of different medical conditions [9]. Since then, this form of compound enriched with oxygen has been applied in nutritional medicine, clinic, treatment of cellular hypoxia, people suffering from migraine, cardiac arrhythmia, ophthalmological and oncological disorders. In addition, therapy was used to improve blood pressure and stimulate the immune system [9]. For dental applications, products based on active oxygen (blue®m) were developed by a team led by Dr. Peter Blijdorp. The company’s main products are oral gel, toothpaste, mouthwash and mouth foam. The products can be used after oral surgeries as adjunct to toothbrushing, to treat gingival inflammation [10], peri-implantitis, among others [11]. Oral topical oxygen therapy (OOT) aims to accelerate the healing process by ensuring angiogenesis, eliminating toxins, collagen synthesis, stimulating the formation of new blood cells, increasing the production of stem cells and bacterial defense [11] [12]. Wound healing is a dynamic process that requires a large amount of oxygen in order to increase cellular metabolic activity [12] and can be divided into phases: inflammatory, proliferative and remodeling [13]. In summary, it involves the coordinated action of populations of resident and migratory cells within the extracellular matrix and cytokines. The biological control of extracellular matrix synthesis by fibroblasts (collagen Types I, III, V, fibronectin and glycosaminoglycans) at the wound site is complex and dependent on growth factors produced by inflammatory cells, keratinocytes and matrix constituents that are present within of the wound [14]. There is evidence that keratinocytes stimulate fibroblasts to synthesize growth factors that in turn will stimulate keratinocyte proliferation in a double paracrine manner [15].
Treatment with OOT is associated with an induction of VEGF expression in wound edge tissue and an improvement in wound size [16], besides improving ischemic wound healing [17]. The positive outcomes obtained with the application of oxygen promote the use of oxygen treatment in the stimulation of wound healing [18]. Thus, the local use of active oxygen as a support for wound healing may be a tendency to replace the use of CLX, due to its side effects. In this way, we sought to pilot study the in vitro effects of blue®m mouthwash on human keratinocytes cell line.
2. Material and Methods
2.1. Reagents and Cell Culture
HACAT (keratinocyte-BCRJ Cat# 0341) cell lines were obtained from the Cell Bank (BCRJ) (Rio de Janeiro, Brazil). HACAT was cultivated on Dulbecco’s modified Eagle’s medium (DMEM - Gibco, Thermo Fischer Scientific, Massachusetts, USA) with high glucose. The medium was supplemented with 10% fetal bovine serum, 1% penicillin, and streptomycin (Gibco, Thermo Fischer Scientific, Massachusetts, USA).
2.2. Analysis of Cell Morphology
The cells morphology were analyses by images captured with a charge coupled device camera (Axiocam mrn, Zeiss, Göttingen, Germany) attached to an inverted microscope (Axio Observer Z1, Zeiss, Göttingen, Germany) using AXIOVISION Software (Zeiss, Göttingen, Germany). All images were analyzed using ImageJ software (http://rsbweb.nih.gov/ij) and panels mounted using Adobe Photoshop® 7 software.
2.3. Cell Proliferation Assay
Cells (HACAT) were cultured in 96-well plates for 12 h. blue®m Mouthwash (Netherlands) was added to the medium at 1, 10 and 100 μl/ml (n = 3), and another well was used as a control, without the presence of antiseptic. After 24 h, 48 h and 72 h cell proliferation was analyzed by CyQUANT® NF Cell Proliferation Assay Kit (Thermo Fischer Scientific, Massachusetts, USA) according to the instructions of the manufacturer. The DNA amount was measured using fluorometer (Spectra Max Gemini XPS) with excitation wavelength of 485 nm and emission wavelength of 530 nm. The mean and standard deviation were calculated using the Microsoft Office Excel (Microsoft Co, Redmond, Washington, USA).
2.4. Statistical Analysis
Statistical analysis was performed with the GraphPad version 5.0 (GraphPad Software, Inc., San Diego, CA, USA). Data were presented as number, percentage, mean, and standard deviation. To compare means between groups, analysis of variance (ANOVA) was performed followed by Bonferroni posttest. The level of statistical significance was 5% (p < 0.05).
3. Results
This is the first in vitro study with blue®m in culture cell lines. Keratinocytes (HACAT) were incubated with different concentrations of blue®m (1, 10 and 100 μl/ml) for 24 h and then submitted to a proliferation assay (Graph 1). It was possible to observe that lower concentration (1 μl/ml) of blue®m increased cell proliferation in HACAT cell lines, while moderate and higher concentrations of the mouthwash present a cytotoxic effect.
The analysis indicate that the proliferation rate of the gingival keratinocytes associated with the mouthwash was significantly higher at 24, 48 and 72 h (1 ul/mL) than in the control group (p = 0.001). The cells subjected to a low concentration of the mouthwash (1 µl/ml) showed an increase in cell proliferation, at intermediate (10 µl/ml) and high (100 µl/ml) concentrations, the mouthwash proved to be toxic, as it was observed that the cells decreased cell proliferation (Graph 1). These results indicate that blue®m has a more pronounced effect in lower concentrations, regarding the proliferation of keratinocytes.
It was possible to observe greater proliferation of keratinocytes when in contact with lower concentrations of the product in the first 24, 48 and 72 hours, showing an increasing with the passing of hours. There is also a change in their morphology presenting a more fusiform characteristic (Figure 1B, Figure 1F, Figure 1J). While in moderate concentrations, the cells died after 48 h (Figure 1G, Figure 1K) and in higher concentrations the cells were detached from the culture plate at 24 h (Figure 1D) and died after 48 h (Figure 1J). These results indicate that the blue®m may have a cytotoxic effect in moderate and higher concentrations.
4. Discussion
Chlorhexidine (CLX) is effective as an antimicrobial agent, and in dentistry it is used mainly for the treatment of gingival conditions and after oral surgeries. However, its cytotoxicity on human cells such as fibroblasts and keratinocytes are described [8] [19]. Therefore, a new product has been brought to use in dentistry with possible antimicrobial, gingival inflammation reduction and healing effects [10] [20], blue®m mouthwash contains active oxygen in its composition, which is known to be closely related to the healing process [12]. Thus, this pilot study aimed to evaluate the effects of this new product on keratinocytes.
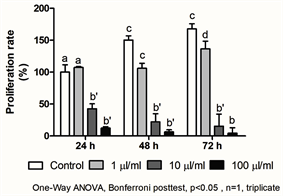
Graph 1. Proliferation rate of keratinocytes submitted to different concentrations of the mouthwash at 24, 48 and 72 hours. Different letters demonstrate statistically significant difference, p = 0.001.
![]()
Figure 1. Cell morphology of the test and control groups. (Bar = 0.14 mm).
Proliferative behavior is a critical feature in relation to cell survival, thus, reliable methods are needed to determine cell proliferation during experiments. Many methods are used to determine proliferation. The nucleic acid content is an indicator that tightly controls the number of cells using the phthalocyanine dye, using the CyQuant test. Cyanine dyes are characterized by low intrinsic fluorescence and major improvements in fluorescence by joining trio nucleic acid. In the test, CyQuant binds to RNA and DNA, but the signal strength of the DNA is up to six times greater than that of an equivalent amount of RNA. The main advantage of cyanine dyes is that it acts independently of changes in cell metabolism. Due to the reliability of the CyQuant test, we used it to determine the cell proliferation of keratinocytes when exposed to the blue®m mouthwash solution.
In this study, the lower the concentration of the solution, the greater the proliferation of keratinocytes. In the area of health studies evaluating cell proliferation is a commonly used methodology. Almeida-Lopez et al. (2001) [21] studying the effects of therapeutic laser on human gingival fibroblasts, demonstrated that the shorter the laser exposure time, the greater the proliferation of these cells. Romero & Delgadillo (2016) [22] submitted epithelial cells to 0.12% chlorhexidine solution for 5, 10, 15, 30 and 1440 minutes. The findings at the end of the 5 minutes already demonstrated that 99.28% of the cells had suffered severe cell damage, making them unfeasible. Previous research has already proposed that CHX inhibits mitochondrial activity and protein synthesis [23], thereby decreasing proliferation, which results in cell death due to ATP40 shortages. Considering this, the use of CHX has similar effects to cigarette smoking on gingival cells as reported by Esfahrood et al. (2015) [24]. Therefore, studies on new rinsing alternatives are necessary to reduce any harmful effect on oral tissues, especially during the post-surgical healing phase.
Other in vitro studies [2] [3] [4] [5] [6] [25] [26] [27], on toxicity of oral mouthwashes, analyzed the proliferation of cells using the MTT test (3- (4,5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide). According to Quent et al. (2010) [28], proliferation in these cases is analyzed indirectly by readings of products derived from cellular metabolism (MTT, Alamar Blue, etc.), therefore, they do not reflect proliferation properly, as cell activity varies greatly during the cell life cycle. In the present study, cell proliferation was analyzed using the CyQuant® method. This method, which is based on DNA quantification, is more accurate when it comes to counting proliferating cells.
There are no other studies that have evaluated the action of this product with active oxygen on the cell proliferation of keratinocytes and thus it is difficult to compare our results. This is just one of the first scientific steps, considered as a pilot study. Much research, both in vitro and in vivo, is needed to complement and better explain the results found here. From a clinical point of view, we can already see an advantage that new products are appearing to minimize or eliminate the effects of other mouthwashes available on the market.
5. Conclusion
This is the first in vitro study with showing that human keratinocytes cell line (HACAT) demonstrated greater proliferation rate when exposed to lower concentrations of blue®m mouthwash, while moderate and higher concentrations of the mouthwash present a cytotoxic effect.