Bio-Therapeutic, Phytochemical Screening and Antioxidant Efficacies of Oyster Mushroom (Pleurotus ostreatus) Obtained from the Wild ()
1. Introduction
Mushrooms are considered as a high nutritional and functional value and they are also accepted as nutraceutical food. Among the various edible species, the Pleurotus ostreatus (oyster mushroom) is one of the most important productions after Agaricus bisporous [1]. The fruiting body of P. ostreatus contains approximately 100 different bioactive compounds, which mainly considered to be a potential new source of dietary fibers, proteins and an abundance of essential amino acids, minerals (Ca, P, K, Fe, Na) and also contain vitamin C, B-complex-thiamine, riboflavin, niacin and folic acid [2]. Due to its documented probiotic properties and relatively high nutritive value, they are recommended in numerous countries as functional foods [3]. Moreover mushrooms have been eaten and appreciated for their flavour, economic and ecological values and medicinal properties for many years [4]. They have ability to reproduce by the recycling of certain agricultural wastes therefore several Pleurotus species are cultivated commercially in various part of world [5].
Cultivation of edible mushrooms is a biotechnological process for lignocellulosic organic waste recycling. It might be the only current process that combines the production of protein-rich food with the reduction of environmental pollution [6]. The origin for cultivation of oyster mushroom P. ostreatus was initiated on experimental basis in Germany by Flack during the year 1917 on tree stumps and wood logs. In India, Cultivation of different varieties of oyster mushroom was initiated in the early sixties and commercial cultivation began in mid-seventies. The main substrate used for cultivation of P. ostreatus is any type of lignocellulose material like paddy straw, wheat straw, corn cobs and hardwoods sawdust, rice hull, etc. [7]. Reports indicate that mushrooms contain many biologically active components that offer health benefits and protection against diseases and are responsible for their antitumor [8], anti-inflammatory, antioxidant [9], and antimicrobial activities [10]. Mushrooms have powerful antioxidant properties derived from compounds such as selenium, ergothioneine and phenolic [9]. In order to utilize this valuable bio-resource better, it is desired to evaluate the antioxidant and antimicrobial activities of P. ostreatus systematically.
Over large number of reports have been published concerning chemical constituents of P. ostreatus and related species. In most of the studies, the nutritional values of mushroom have been offered as in dried fruit bodies. Generally, fresh Pleurotus mushroom contain 85% - 95% moisture. The fruiting body of P. ostreatus contains approximately 100 of different bioactive compounds, which mainly considered as a potential new source of dietary fiber. Whereas, fungal cell wall are rich in non-starch polysaccharides, of which β-glucan are most interesting functional components and phenolic compounds such as protocatechuic acid, gallic acid, homogentisic acid, rutin, myrictin, chrysin, naringin, tocopherol like α-tocopherol and γ-tocopherol, ascorbic acid and β-carotene of each having their own outstanding medical effects [11]. Moreover, they are healthy foods, rich in protein, lipids, carbohydrates, vitamins and minerals content but low in calories and fat content [12].
Oxidative stress has been implicated as a primary factor in the progression of many degenerative diseases like cancer and hepatotoxicity. Nevertheless, antioxidants such as phenolic and flavonoid compounds are delaying and inhibiting oxidative processes. Generally, Pleurotus mushrooms are rich in vitamin and selenium content which are the important natural antioxidants in biological systems [13]. It was reported that, an extract of P. ostreatus enhanced the Catalase gene expression and decreased the incidence of free radical-induced protein oxidation in aged rats, thereby protecting the occurrence of age-associated disorders that involve free radicals [13]. The ethanolic extract of the oyster mushroom P. ostreatus are reported to have potent antioxidant activity in both in vitro and in vivo. The ethanolic extract exhibit in vitro antioxidant activity by virtue of its scavenging hydroxyl and superoxide radicals, inhibiting lipid peroxidation, reducing power on ferric ions, chelating ferrous ions and quenching 2,3-diazabicyclo[2,2,2]oct-2-ene (DBO). It also exhibits as a good in-vivo antioxidant activity by reducing the intensity of lipid peroxidation and by enhancing the activities of enzymatic and non-enzymatic antioxidants [13].
Pleurotus ostreatus have been eaten and appreciated for their flavour, economic and ecological values and medicinal properties for many years and have several beneficial properties. Therefore, it is important to evaluate the possible mechanism of action by which Pleurotus ostreatus achieve its inhibitory and antioxidant effects by studying the interaction of its extracts and determine its biological inhibitory effect as an inhibitor. Therefore, the specific objectives of this research work are to evaluate the antibacterial potential and the antioxidant properties of Pleurotus ostreatus obtained from the wild.
2. Materials and Methods
2.1. Collection and Identification of Mushroom Samples
Pleurotus ostreatus used for this research work was obtained on the 23rd of September, 2019 from a local farm in Odigbo and Okitipupa in the Southern Senatorial Distrinct where the University is located. Identification and authentication were carried out at the Research Laboratory in the Department of Biological Sciences of Ondo State University of Science and Technology, Okitipupa. The mushrooms were prepared using [14] method and was later oven dried for 24 hr at 45˚C. The dried samples were pulverized using industrial blender (Kenwood BL335B).
2.2. Preparation of Samples
The collected sample was oven dried for 24 hr at 45˚C and pulverized, packaged in air tight containers, stored at ambient condition for subsequent analysis. The aqueous extract of the sample was gotten after soaking 2.0 g of sample in water and placed in speed governing multi-purpose oscillator for 2 hours, filtered and centrifuged. The supernatant obtained after filtering using muslin cloth was centrifuged at 3500 revolutions at 25˚C for 1 hr to obtain a clearer extract which was used to determine the total phenolic content, total flavonoids and other antioxidant activities.
For antimicrobial, 52.0 g of the mushroom was soaked in 300 ml of methanol for three days, stirred at intervals, filtered using muslin cloth and the filtrate was passed through a rotary evaporator (Rotavapor BÜCHI R-200 with heating bath B-490, Büchi, Konstanz, Germany) subsequently exposed to the atmosphere to evaporate the solvent from the mushroom extract for antimicrobial activities.
2.3. Collection of Test Organisms
Pure cultures of some Clinical isolates were used for the assay. The test organisms are: Staphylococcus aureus, Psedomonas aeruginosa, Escherichia coli and Klebsiella pneumonia which were obtained from the State Specialist Hospital, Okitipupa, Ondo State. The isolates were later resuscitated in nutrient agar and maintained at 4˚C for further use.
2.4. Evaluation of Antimicrobial Activity
Agar well diffusion techniques as described by [15], was adopted for the study. Mueller Hinton agar plates (MHA Oxoid), were inoculated with 0.1 mL of an overnight broth culture of each bacteria isolate (Equivalent to 106 Cfu/ml) MF (McFarland standard) in sterile Petri-dish using sterile swab sticks. Holes were bored on the plates by using standard sterile cork borer of 6 mm diameters and equal volumes of the extract with vancomycine and amicosin as positive control were transferred into the well with the aid of micropipette. The plates were allowed to stand for one hour at 25˚C ± 2˚C to allow for proper diffusion of the extract. The plates were incubated at 37˚C for 24 - 48 hrs until marked decline in the potency of the mushroom extract to inhibit the growth of the test isolates was observed. Zone of inhibitions were measured in millimeter (mm) and the values were calculated and recorded.
2.5. Antioxidant Assay
2.5.1. Determination of Total Phenolic Content
The total phenolic content was determined by using Folin-Ciocalteu colourimetric method based on oxidation-reduction reaction [16]. Appropriate dilutions of Pleurotus ostreatus extracts were oxidized with 500 μl of 10% Folin-Ciocalteau’s reagent (v/v) and neutralized by 400 μl of 7.5% sodium carbonate. The reaction mixture was incubated for 45 mins at 45˚C and the absorbance was measured at 765 nm against the blank in the UV-Visible spectrophotometer. A mixture of 100 μl of distilled water, 500 μl of 10% Folin-Ciocalteau’s reagent and 400 μl of 7.5% sodium carbonate serves as the blank. The total phenol content was subsequently calculated as gallic acid equivalent.
2.5.2. Determination of Total Flavonoid Content
The total flavonoid content was determined using a method by [17]. The appropriate dilutions of the sample were mixed with 500 μl of aqueous extract, 50 μl 10% of AlCl3, 50 μl of 1 M potassium acetate. Subsequently was allowed to incubate at 25˚ ± 2˚ for 30 mins. The absorbance of the reaction mixture was subsequently measured at 415 nm against the blank in the UV-Visible spectrophotometer; the total flavonoid content was subsequently calculated as quercetin equivalent.
2.5.3. Determination of Reducing Power (FRAP)
The reducing property of aqueous extracts of Pleurotus ostreatus extracts were determined by assessing the ability of the extracts to reduce FeCl3 solution as described by [18]. Different dilutions of aliquots were mixed with different dilutions of sodium phosphate buffer (pH 6.6), 250 μl of potassium ferricyanide and 250 μl of distilled water. The mixture was incubated at 50˚C for 20 mins and then 250 ml of 10% trichloroacetic acid was added. This mixture was mixed with 1 ml of water and 200 μl of ferric chloride for the different dilutions of the extracts. The absorbance was measured at 700 nm against the blank. A solution of 250 μl of PO4 (pH 6.6), 250 μl of KfeCN4, 250 μl of distilled water, 250 ml of 10% trichloroacetic acid, 1 ml of water and 200 vml of ferric chloride serves as the blank.
2.5.4. 2,2-diphenyl-1-picrylhydrazyl (DPPH) Free Radical Scavenging Ability
The free radical scavenging ability of the extract against DPPH (2,2-diphenyl-1-picrylhydrazyl) free radical was evaluated as described by [19]. Appropriate dilution of the extracts were mixed with distilled water (200 - 500 µl) was mixed with 600 μl of 0.4 mM methanolic solution containing DPPH radicals, the mixture was left in the dark for 30 mins at room temperature and the absorbance was taken at 516 nm against distilled water blank. The DPPH free radical scavenging ability was subsequently calculated.
2.5.5. Determination of ABTS Radical Scavenging Ability
The ABTS scavenging ability of the extract was determined according to the method described by [20]. The ABTS was generated by reacting an (7 mmol/l) ABTS aqueous solution with K2S2O8 (2.45 mmol/l, final concentration) in the dark for 16 hrs at room temperature and adjusting the absorbance of 734 nm to 0.700 with ethanol. Appropriate dilutions of the extract were mixed with distilled water and 900 μl of ABTS solution. After incubation in the dark for 15 mins, absorbance was measured at 734 nm against distilled water blank. The trolox equivalent antioxidant capacity (TEAC) was subsequently calculated using trolox as the standard.
2.6. Statistical Analysis
All the experiments for determination of total phenolics, total flavonoids, and antioxidant properties using DPPH and cellular antioxidant assay (CAA) were conducted in triplicates. The values are expressed as the mean ± standard deviation (SD). Average crop yield was calculated by dividing total yield by number of plants grown [21]. The results of the replicates were pooled and expressed as mean ± standard deviation (S.D) values. Analysis of variance (ANOVA) was performed on data sets and significant differences (p < 0.01 and p < 0.05) between the means were determined by Duncan’s range test [22].
3. Results
3.1. Antibacterial Activities
The Antibacterial activities of Pleurotus ostreatus extract are shown in Plates 1-3 while Table 1 equally shows the antibacterial activities of Pleurotus ostreatus
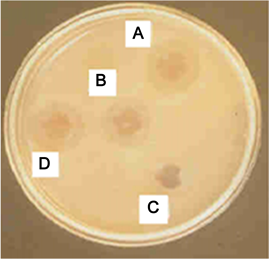
A—well containing standard antibiotics (Amicosin); B—well containing (APO) Aqueous extract of Pleurotus ostreatus; C—well containing standard antibiotics (Vancomycine); D—well containing (MetPO) Methanolic extract of Pleurotus ostreatus.
Plate 1. Inhibition of Psedomonas aeruginosa by the extracts of Pleurotus ostreatus and standard antibiotics in agar well diffusion assay.
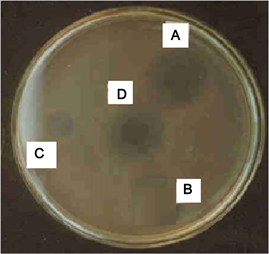
A—well containing standard antibiotics (Amicosin); B—well containing (APO) Aqueous extract of Pleurotus ostreatus; C—well containing standard antibiotics (Vancomycine); D—well containing (MetPO) Methanolic extract of Pleurotus ostreatus.
Plate 2. Inhibition of Klebsiella pneumonia by the extracts of Pleurotus ostreatus and standard antibiotics in agar well diffusion assay.
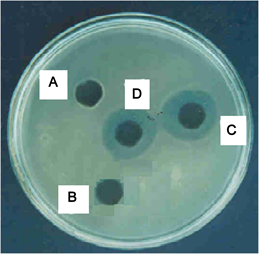
A—well containing standard antibiotics (Amicosin); B—well containing (APO) Aqueous extract of Pleurotus ostreatus; C—well containing standard antibiotics (Vancomycine); D—well containing (MetPO) Methanolic extract of Pleurotus ostreatus.
Plate 3. Inhibition of Staphylococcus aureus by the extracts of Pleurotus ostreatus and standard antibiotics in agar well diffusion assay.
![]()
Table 1. Antibacterial activities of Pleurotus ostreatus.
Mean ± standard deviation ±SD of triplicate determination. Samples carrying the same superscripts in the same row are not significantly different at (p > 0.05). Keys: APO: Aqueous extract of Pleurotus ostreatus; MetPO: Methanolic extract of Pleurotus ostreatus.
extract and the positive control (Vancomycine and Amicosin) against Gram-positive (Staphylococcus aureus) and Gram-negative (Psedomonas aeruginosa, Escherichia coli and Klebsiella pneumonia) bacteria been evaluated by the use of standard zone of inhibition.
3.2. Antioxidant Assay
3.2.1. Phenolic and Flavonoid Composition and ABTS Scavenging Ability and Reducing Power (FRAP)
Table 2 shows the total phenolic and flavonoid contents of the mushroom extract and the total phenolic content reported as gallic acid equivalent was 12.55 mg GAE/g while the total flavonoid content reported as quercetin equivalent was 7.22 mg QE/g. The ABTS scavenging ability reported as Trolox equivalent antioxidant capacity (TEAC) of the mushroom and the result revealed that Pleurotus ostreatus scavenged ABTS of a mean value of 1.99 mmol TEAC/100g and its reducing power is 5.25 mg/g.
3.2.2. 2,2-diphenyl-1-picrylhydrazyl (DPPH) Free Radical Scavenging Ability
Figure 1 shows the DPPH free radical scavenging ability of the aqueous extract
![]()
Table 2. Total phenolic and flavonoid contents, FRAP and ABTS scavenging ability of aqueous extract of Pleurotus ostreatus.
Values represent mean ± standard deviation of replicate experiments. Samples carrying the same superscripts in the same row are not significantly different at (p > 0.05).
![]() Key: PO—Pleurotus ostreatus.
Key: PO—Pleurotus ostreatus.
Figure 1. DPPH free radical scavenging ability of aqueous extract of Pleurotus ostreatus.
of Pleurotus ostreatus and the extracts scavenged DPPH radical in a dose-dependent manner (0 - 13.33 mg/ml).
4. Discussion
Pleurotus ostreatus represents one of the greatest untapped resources of nutritious food. Several species of Pleurotus are primarily consumed for their nutritive value and used industrially as a bioremediator [23]. The present study has further revealed the antibacterial potential of P. ostreatus. Extracts of the fruiting body prepared in different solvents showed antibacterial activity (zone of inhibition in the range varied range) against several pathogenic microorganisms of clinical implications. Similar results have been reported in earlier studies [24] [25].
The secondary metabolites from Pleurotus ostreatus exert antimicrobial activity through different mechanisms. Nowadays, the use of antibiotics increased significantly due to infections causing life-threatening illness and the pathogenic bacteria becoming resistant to drugs in common due to its discriminate use of antibiotics [26]. It becomes a greater problem of giving treatment against resistant pathogenic bacteria. While decreased efficiency and resistance to pathogen to antibiotic has necessitated the development of new alternatives [27]. The present study observed that methanolic extract of Pleurotus ostreatus showed higher antibacterial activity against E. coli, S. aureus, P. aeuroginosa, K. pneumonia than the aqueous extract which showed little or no inhibition (Table 1) indicating the microorganisms are susceptible to the methanolic extract of Pleurotus ostreatus while for the aqueous extract of Pleurotus ostreatus, the microorganisms are resistant except Pseudomonas aeruginosa which shows zones of inhibition of 8.00 ± 1.00 mm, which makes P. aerginosa susceptible to aqueous extract of Pleurotus ostreatus. Vancomycine was used as positive control for gram positive bacteria while amicosine for gram negative bacteria. Vancomycine shows more zone of inhibition against S. aureus compared to the extract but was non-reactive to the gram negative bacteria. Subsequently, gram negative bacteria were susceptible to amicosin and shows higher zones of inhibition than the extract but S. aureus was not susceptible to amicosin (Plate 1 and Plate 2). The variation in the effectiveness of the extract against different microorganism depends upon the membrane permeability of the microbes for the extracts and their metabolism. The data obtained from the well diffusion method revealed that P. ostreatus exhibit antibacterial activity this could be due to presence of polysaccharides, alkaloids, steroids and terpenoids. The results also lined up with [28] postulated that organic extract (methanol and chloroform) of P. osteatus has been manifested as effective against Gram-positive bacteria which showed to be a potential source of antibacterial agents [28]. Also, it is interesting to note that the pathogenic microorganism, Pseudomonas aeruginosa, which is resistant to conventional synthetic antibiotics like gentamycin and tetracycline was found to show susceptibility to the aqueous extracts this is because mushrooms produce various antiviral, antifungal compounds to survive in the wild against competing or pathogenic agents [29].
The antioxidant capacity is a way of depicting the effect of reducing compounds in the mushroom extract. The observed total antioxidant activity can be attributed to the presence of phytochemicals. Antioxidants compounds exert their effects through different mechanisms such as inhibiting hydrogen abstraction, binding transition metal ions, radical scavenging and disintegrating peroxides [30].
In order to confirm the antioxidant potential of aqueous extract of P. ostreatus mushroom, five different antioxidant testing systems were employed such as ABTS, DPPH, FRAP, total flavonoid and phenolic. The inhibitory effect of Pleurotus ostreatus was attributed to the presence of phenolic and flavonoid compounds extract in which the phenolic and flavonoid assay shows a high value of 12.55 mg GAE/g and 7.22 mg QE/g (Table 2). The ABTS assay is based on the inhibition of the absorbance of the radical cation ABTS+, which has a characteristics long wavelength absorption spectrum [31]. The result of the ABTS+ scavenging ability of Pleurotus ostreatus as trolox equivalent antioxidant capacity (TEAC) of mean value 1.96 mmol TEAC/100g revealed that the extracts of Pleurotus ostreatus has ABTS scavenging ability as shown in Table 2. DPPH being a stable free radical accepts electrons or hydrogen radicals to become stable diamagnetic molecules [32]. The reaction of aqueous extract of Pleurotus ostreatus with purple colored DPPH radical is to reduce the stable DPPH radicals to diphenyl picrylhydrazine due to the extract antioxidant property. The degree of discoloration indicates the antioxidants potential of aqueous extract of pleurotus ostreatus based on its ability to convert the DPPH radicals to DPPH-H, the non-radical reduced form of the DPPH radicals upon hydrogen donation by the antioxidants present in the extracts as shown in Figure 1 [33]. The results obtained from this study are in line with the finding of [34]. Reducing power is a novel antioxidant defense mechanism; the mechanisms available to affect this property are by electron transfer and hydrogen atom transfer [35], this is because the ferric-to-ferrous ion reduction occurs rapidly with all reductants with half reaction reduction potentials above that of Fe3+/Fe2+. The values in the ferric reducing antioxidant property (FRAP) assay will express the corresponding concentration of electron-donating antioxidants [35]. Antioxidants are strong reducing agents and this is principally because of the redox properties of their hydroxyl groups and the structural relationships of any parts of their chemical structure [36]. The ability of aqueous extract of Pleurotus ostreatus extract to reduced Iron (III) to Iron (II) indicates its redox properties.
5. Conclusion and Recommendation
The comprehensive information made available by this study shows that the tested oyster mushroom extract possesses many promising therapeutic properties at varying amounts. Based on the results obtained from the analysis, it can be concluded that the methanolic extract of this edible mushroom (Pleurotus ostreatus) possessed a broad-spectrum antibacterial activity and thus the potential of developing antimicrobials from them appears rewarding. They can also be used as functional foods (serve as a rich source of natural rich antioxidant food for the enhancement of the immune system against oxidative damage) since they have significant antioxidant activity and they can be used as easily accessible source of natural antioxidants and as possible food supplement and in pharmaceutical industry considering the lingering threat of multi-drug resistance.
Acknowledgements
Ondo State University of Science and Technology is hereby acknowledged for providing a conducive environment for this research work to be carried out.