Risk Factors Affecting Ischemic Stroke: A Potential Side Effect of Antihypertensive Drugs ()
1. Introduction
Stroke is a worldwide health problem, the world’s second-leading cause of death and third-leading cause of disability [1]. Stroke occurs when something blocks the blood supply to part of the brain or when a blood vessel in the brain bursts [2]. As the World Health Organization (WHO) points out [3], “Annually, 15 million people worldwide suffer a stroke. Of these, 5 million die and another 5 million are left permanently disabled, placing a burden on family and community.” The WHO estimates that stroke caused 5781 thousand deaths in 2016 [4]. The World Stroke Organization (WSO) [5] estimates the occurrence of 13.7 million new strokes, 5.5 million deaths, and 116 million healthy years of life lost per year. It is also estimated that 80 million stroke survivors worldwide exist, and that one in four people over the age of 25 will experience stroke in their lives. Lindsay et al. [6] reported additional details, namely that there were 13,676 thousand (185.1 per 100,000 people) stroke incidents and 5528 thousand deaths in 2016. Virani et al. [7] described that the mean global lifetime risk of stroke increased from 22.8% in 1990 to 24.9% in 2016. Benjamin et al. [8] found that there were 6.5 million stroke deaths globally, making stroke the second-leading cause of death in 2013.
In the United States, 4.34% of people answered that they had had a stroke at some point in time [9]. Approximately 795,000 strokes occur each year, and their estimated cost averaged $33.9 billion annually from 2012 to 2013 [8] [10]. A total of 2,813,503 deaths were reported, and stroke was the fifth-leading cause of death, killing 146,383 people in 2017 [11]. Also, stroke reduces mobility in more than half of stroke survivors aged 65 and over in the United States [8]. For research on strokes in other countries see the review work of Thrift [12].
The disruption of the blood supply may result from either blockage (ischemic stroke) or the rupture of a blood vessel (hemorrhagic stroke) [13]. Currently, the majority of strokes are ischemic strokes. Mozzafarian et al. [14] reported that 87% of strokes are ischemic strokes. Lindsay et al. [6] reported that there were 9,556,444 and 4,120,318 ischemic and hemorrhagic strokes worldwide in 2016, respectively.
In Japan, the medical cost of cerebrovascular diseases was 1.809 trillion yen in fiscal year 2017 [15]. Cerebrovascular diseases caused 108,186 deaths, making them the fourth-leading cause of death, and they accounted for 8.9% of total (1,362,470) deaths in 2018 [16]. Among those deaths, 45,043 were due to hemorrhagic stroke (33,047 cerebral hemorrhages and 11,996 subarachnoid hemorrhages), and 60,365 were due to ischemic stroke. The number of patients treated at hospitals or clinics on the survey day (one selected day in October) for cerebrovascular diseases was 231.9 thousand, and the number treated for ischemic stroke was 150.8 thousand; the total number of cerebrovascular disease patients was estimated to be 1.12 million in 2017 [17].
Although ischemic and hemorrhagic strokes damage the brain cells, their mechanisms and treatment methods are quite different. Therefore, they should not be included in the same category. Ischemic stroke happens when blood flow through the artery that supplies oxygen-rich blood to the brain becomes blocked. Blood clots often cause the blockages that lead to ischemic stroke [13]. The majority of stroke patients were ischemic stroke patients. Therefore, this study focuses on ischemic stroke. The American Heart Association (AHA) Stroke Council has provided guidelines [18] [19] addressing prehospital care, urgent and emergency evaluation and treatment with intravenous and intra-arterial therapies, and in-hospital management for the early management of acute ischemic stroke. Reperfusion with tissue plasminogen activator remains the gold standard treatment for ischemic stroke within 4.5 hours (especially 3 hours) of stroke onset [20]. Numerous studies have been done on ischemic stroke treatments [21] - [32] and rehabilitation [33] - [40].
The risk factors for stroke are classified as nonmodifiable (cannot be controlled) and modifiable (can be controlled). Age, sex, race/ethnicity, family history, prior stroke, transient ischemic attack (TIA), and heart attack are nonmodifiable risk factors. High blood pressure (hypertension), smoking, diabetes, diet, physical inactivity, obesity, high blood cholesterol, carotid artery disease, peripheral artery disease, atrial fibrillation and other heart disease, and sickle cell disease are commonly reported to be modifiable risk factors [41] [42] [43].
Regarding ischemic stroke, Allen and Bayraktutan [44] reported hypertension, diabetes mellitus, smoking, coronary heart disease, atrial fibrillation, left ventricular hypertrophy, waist-to-hip ratio, lipoprotein, von Willebrand factor, white blood cell count, C-reactive protein, homocysteine, and reaction oxygen species/oxidative stress as modifiable factors; and age, sex, and race as nonmodifiable factors. They also described that stroke events could be reduced by approximately 80% through lifestyle modifications. Among the modifiable risk factors, Fahimfar et al. [45] concluded that chronic kidney disease, hypertension, and diabetes were the strongest independent predictors of stroke based on a 9-year follow-up study in Iranian subjects. Ohia et al. [46] analyzed the risk factors for ischemic stroke subtypes using the dataset obtained from the Atherosclerosis Risk in Communities (ARIC) study. Between 1987 and 1989, 14,448 men and women took part in the first examination of the ARIC study. During the average follow-up of 13.4 years, 541 ischemic stroke incidents (105 lacunar, 326 non-lacunar, and 100 cardioembolic) occurred. Although they concluded that “The impact of traditional and nontraditional risk factors other than hypertension on the incidence of ischemic stroke varied according to its subtype,” current smoking and diabetes mellitus were significant risk factors in all their estimated results. Bang et al. [47] also reported nontraditional risk factors.
In this paper, factors that might affect ischemic stroke are reexamined using the probit and ordered probit models.
2. Data and Methods
2.1. Data
Japan has a public health insurance system and all citizens belong to some type of public health insurance organization. Most employees aged 40 or over are required to undergo medical checkups at least once a year by the Industrial Safety and Health Act [48], and their family members can also receive medical checkups on a voluntary basis. The dataset was created with the cooperation of three health insurance societies. The monthly reports of all medical treatments and payments, called “receipts,” are sent from medical institutes to the health insurance societies. The original dataset contained 175,123 medical checkups and 6,312,125 receipts obtained from April 2013 to February 2017 (the Japanese fiscal year starts in April and ends in March of the next year). The diseases classified as ischemic stroke were chosen according to the classification of the Ministry of Health, Labour and Welfare [49] (code number: 0906, International Disease Classification 10th Revision (ICD 10) code number: I63). To prevent the disease, it is necessary to know who exhibits risk factors in advance and to give them proper lifestyle modification and medical guidance. Therefore, I analyze the data of people who were not treated for ischemic stroke in fiscal year t, and checked whether they were treated for ischemic stroke in the next fiscal year (i.e., fiscal year t + 1) or not based on a survey of receipts.
2.2. Probit and Ordered Probit Models
The probit and ordered probit models are used in the analysis. We let
be a dummy variable taking 1 if person i received ischemic stroke treatment in fiscal year t and 0 otherwise. I consider the observations satisfying
and perform an analysis using the probit model:
,
(1)
if
and
if
,
where
is the conditional probability that
,
is a vector of explanatory variables,
follows the standard normal distribution,
is the distribution function of the standard normal distribution, and
is a latent variable.
The seriousness of the disease differs by patient and must be considered. The receipts are monthly reports from medical hospitals (including clinics). If a patient goes to one hospital in a given month, it is summarized as one receipt. If a patient goes to the same hospital in two months or more, the health insurance society receives different receipts depending on the number of months. When a patient goes to different hospitals within the same month, the health insurance society receives different receipts depending on the number of hospitals. It is reasonable to assume that if a treatment ends in a short period at one hospital, the condition of the patient is not very serious. (In a serious situation, a patient might stay at the hospital for a long period or might be sent to large specialized hospitals.) Among a total of 170,123 observations, 170,870 were not treated as ischemic strokes, 2084 went to one hospital for one month, and 2169 went to multiple hospitals or went to the hospital in multiple months.
Let
be the total number of hospitals and months in which the patient i was treated for ischemic stroke in fiscal year t+1 (i.e., if patient i goes to
different hospitals and for
months to hospital j,
). It might be possible to assume that the condition of a patent is relatively mild if
(the patient goes to just one hospital for only one month to treat ischemic stroke in a fiscal year). Therefore, to evaluate the seriousness of the patient’s condition, I consider the ordered probit model, given:
,
(2)
if
,
if
, and
if
,
and
.
and
are threshold values, and
does not contain a constant term. For the details of the model, see Amemiya [50].
3. Explanatory Variables and Estimated Models
3.1. Explanatory Variables
Several different models and variables are considered. The nonmodifiable explanatory variables include: Age, Female (1: if female; 0: otherwise), Height (cm), Cerebrovascular (diagnosed previously with some type of cerebrovascular disease: 1; 0: otherwise), Cardiovascular (diagnosed previously with some type of cardiovascular disease (CVD): 1; 0: otherwise), Kidney (diagnosed previously with some type of kidney disease: 1; 0: otherwise), Diabetes (getting diabetes treatments; 0: otherwise), F_year14 (1: fiscal year 2014; 0: otherwise), and F_year15 (1: fiscal year 15; 0: otherwise).
The following variables are used as modifiable explanatory variables: BMI (=height (m)/weight (kg)2), SBP (systolic blood pressure, mmHg), DBP (diastolic blood pressure, mmHg), HDL (high density lipoprotein cholesterol blood, mg/dL), LDL (low-density lipoprotein cholesterol, mg/dL), Triglyceride (mg/dL), GGP (γ-glutamyl transferase, units per liter: U/L), AST (aspartate aminotransferase, U/L), ALT (alanine aminotransferase, U/L), B_Sugar (blood sugar, mg/dL), HbA1c (hemoglobin A1c, %), U_Sugar (urine sugar integers of 1 - 5, judged by colors of reagent; 1:undetected, 2: around 50 mg/dL, 3: around 100 mg/dL, 4: around 250 mg/dL and around 500 mg/dL or over; 1 is normal, 5 is worst), U_Protein (integers of 1 - 5; judged by colors of reagent, 1:undetected, 2: around 15 mg/dL, 3: around 30 mg/dL, 4: around 100 mg/dL and around 250 mg/dL or over; 1 is normal, 5 is worst), Weight_1(weight changed by 3 kg or more in a year), Weight_20 (weight increased by 10 kg or more from age 20), Antihypertensive (1:taking antihypertensive drugs, 0: otherwise), Glycemic (1: taking glycemic drugs; 0: otherwise), Lipid (1: taking lipid drugs; 0: otherwise), Eat_Fast (1: eating faster than other people; 0: otherwise), Late_Supper (1: eating supper within two hours before bedtime three times or more in a week; 0: otherwise), Night_Snack (1: eating late-night snacks after supper three times or more in a week, 0: otherwise), No_breakfast (1: not eating breakfast three times or more in a week; 0: otherwise), Exercise (1: doing exercise for 30 minutes or more twice or more in a week for more than a year; 0 otherwise), Activity (1: doing physical activities (walking or equivalent) for one hour or more daily, 0: otherwise), Speed (1: walking faster than other people of a similar age and the same gender; 0: otherwise), Alcohol_freq (0: not drinking alcoholic drinks, 1: sometimes, 2: everyday), Alcohol_amount (0: not drinking; 1: drinking less than 180 ml of Japanese sake wine (with an alcohol percentage of about 15%) or equivalent alcohol in a day when drinking; 2: drinking 180 - 360 ml; 3: drinking 360 - 540 ml; 4: drinking 540 ml or more), Smoke (1: smoking; 0: otherwise), and Sleep (1: sleeping well; 0: otherwise).
3.2. Estimated Models
Since some variables representing life style (from Weight_1 to Sleep) are not available from one health insurance society, I consider Models A and B for the probit model and Models C and D for the ordered probit model. Models A and C were the probit and ordered probit models that contained all explanatory variables including variables representing life style but the data from one health insurance society were not used. On the other hand, Models B and D were the pobit and ordered probit models that contained the data from all health insurance societies but some variables representing life style were not used.
1) Probit model (dependent variable: ISit+1)
Model A:
Model B:
2) Ordered probit model (dependent variable: Nit+1).
Model C:
Model D:
4. Results of Estimation
Observations with missing values or taking extremely strange values (SBP: over 300 or under 30; DBP: over 200 or under 30; DBP larger than SBP; HDL: over 500; LDL: over 500; Triglyceride over 1000; and B_Sugar over 500) were excluded. 50,542 observations, including 49,827: (
,
), 715: (
,
), 534: (
,
) and 181: (
,
), were used for Models A and C. A total of 59,341 observations, including 58,496: (
,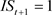 ), 845: (
), 845: (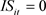 ,
,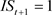 ), 626: (
), 626: (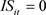 ,
,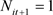 ) and 219: (
) and 219: (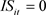 ,
,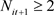 ), were used for Models B and D. The summaries of explanatory variables are given in Table 1 and Table 2.
), were used for Models B and D. The summaries of explanatory variables are given in Table 1 and Table 2.
The results of the estimation are given in Tables 3-6. Table 3 shows the results of Model A, the probit model containing all variables with 50,402 observations. Table 4 shows the results of Model B, the probit model with 58,496 observations. Table 5 shows the results of Model C, the ordered probit model containing all variable with 50,402 observations. Table 6 shows the results of Model D, the ordered probit with 58,496 observations. We obtained similar results in all models. All models contained all nonmodifiable variables. Among these variables, the estimates of Age, Female, and Cerebrovascular were positive and significant at the 1% significance level; and the estimates of Cardiovascular were positive and significant at the 5% level. However, the estimates of the other nonmodifiable variables were not significant even at the 5% level in all four models. This implies that Age, Female, Cerebrovascular and Cardiovascular
![]()
Table 1. Summary of explanatory variables (Models A and C).
SD: Standard deviation; 50542 observations.
![]()
Table 2. Summary of explanatory variables (Models B and D).
SD: Standard deviation; 59,341 observations.
![]()
Table 3. Results of Estimation (Model A).
SE: Standard error; *: Significant at the 5% level; **: Significant at the 1% level. 50,542 observations; 49,827: (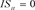 ,
, ), 715: (
), 715: (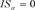 ,
, ).
).
![]()
![]()
Table 4. Results of Estimation (Model B).
SE: Standard error; *: Significant at the 5% level; **: Significant at the 1% level. 59,341 observations; 58,496: (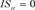 ,
, ), 845: (
), 845: (![]() ,
,![]() ).
).
![]()
![]()
Table 5. Results of Estimation (Model C).
SE: Standard error; *: Significant at the 5% level; **: Significant at the 1% level. 50,542 observations; 49,827: (![]() ,
,![]() ), 534: (
), 534: (![]() ,
,![]() ), 181: (
), 181: (![]() ,
,![]() ).
).
![]()
Table 6. Results of Estimation (Model D).
SE: Standard error; *: Significant at the 5% level; **: Significant at the 1% level. 59,341 observations; 58,496: (![]() ,
,![]() ), 626: (
), 626: (![]() ,
,![]() ) and 219: (
) and 219: (![]() ,
,![]() ).
).
were important factors to prevent ischemic stroke. In other words, the special care should be necessary to people with these risk factors for proper treatments of the disease.
For modifiable variables, BMI, SBP, DBP, HDL, Triglyceride, B_sugar, HBA1c, U_Sugar, U_Protein, GTP, AST, ALT, Smoke, Antihypertensive, Glycemic and Lipid were contained in all models. Among these variables, the estimates of Antihypertensive were positive and significant at the 1% level in all models. Weight_1, Weight_20, Eat_Fast, Late_Supper, Night_Snack, No_Breakfast, Alcohol_freq, Alcohol_amount and Sleep were contained in Models B and D. The estimates of Weight_1 were positive and those of Sleep were negative and significant at the 1% level in both models. The variables are important risk factors for ischemic among modifiable factors.
The estimates of the other nonmodifiable and modifiable variables were not significant at the 5% level, except SBP in Model C and Lipid in Model B (positive and significant at the 5% level), and Smoke in Model B and BMI in Model D (negative and significant at the 5% level).
5. Discussion
Among the nonmodifiable variables, age, gender, and history of cerebrovascular diseases are considered to be important risk factors for ischemic stroke as pointed out by previous studies. The history of cerebrovascular diseases is considered to be an especially important factor. The t-values of Cerebrovascular were 7.75 - 9.25, significant at any reasonable level, and the estimated values were much larger than those of the other variables in all models. The American Stroke Association (ASA) [42] stated that “A person who’s had one or more transient ischemic attacks (TIAs) is almost 10 times more likely to have a stroke than someone of the same age and sex who hasn’t.” The results of the present study conform with this statement from the ASA. In the case of ischemic stroke treatments, time is the most critical factor [20], and it is very important to ensure that medical personnel know a patient’s history of cerebrovascular diseases. It is essential that not only the patient but also the people around the patient, including family members, colleagues, friends and family doctors, should share this information to help determine the treatment.
Among the modifiable variables, taking antihypertensive drugs, recent large weight change (more than 3 kg within a year), and sleeping well are considered to be important factors. Among these variables, recent large weight change and sleeping well had the expected results; the former increased and the latter decreased ischemic stroke risks. However, taking antihypertensive drugs did not. Hypertension was previously considered an important risk factor [41] - [46]. In this study, the estimates of SBP and DBP became significant at the 5% level just in one model; besides, taking antihypertensive drugs had a negative impact at the 1% level in all four models. Although this seems to contradict the results of previous studies, the results of Ohira et al. [46] showed that taking antihypertensive drugs was a significant risk factor in ischemic stroke subgroups as measured by rate ratios except in one case. The results of this study are consistent with theirs.
In November, 2017, the American College of Cardiology (ACC), AHA, and nine other organizations presented the “2017 ACC/AHA/AAPA/ABC/ACPM/ AGS/APhA/ASH/ ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults” (hereafter the 2017 ACC/AHA Guideline) [51]. In the 2017 ACC/AHA Guideline, the new criterion for hypertension was 130/80mmHg, lowered from the conventional one of 140/90mmHg. However, other organizations such as the American Academy of Family Physicians [52], the European Society of Cardiology and European Society of Hypertension [53], Hypertension Canada [54] [55], American Diabetes Association [56], and the Japanese Society of Hypertension [57] decided to maintain the diagnostic criterion of 140/90mmHg for the general public.
The 2017 ACC/AHA Guideline gave substantial weight to the Systolic Blood Pressure Intervention Trial (SPRINT) [58]. The SPRINT was a trial involving 9361 participants with an SBP of 130 mmHg or higher and increased CVD risks but without diabetes. The participants were randomly assigned to two groups; one was the standard treatment group with an SBP target of 140 mmHg or less; and the other was the intensive treatment group with an SBP target less than 120 mmHg. The average SBP at the beginning of the trial was 139.7 mmHg in both groups. In the trial, the average numbers of antihypertensive drugs given to participants were 1.8 and 2.8, and the reductions of SBP were 5.1 mmHg and 18.2 mmHg in the standard and intensive treatment groups, respectively. Nawata, Sekizawa, and Kimura [59] reported that taking antihypertensive drugs would reduce SBP by 9.2 mmHg. In this study, SBP became a significant risk only in one model (Model C). With that model, the benefits of the reduction of SBP were 0.0156, 0.0281 and 0.057 for the 5.1, 9.2 and 18.2 mmHg reduction cases, respectively. On the other hand, the negative side effect of taking antihypertensive drugs was 0.121, which was much larger than the benefits. Major antihypertensive drugs include: angiotensin converting enzyme (ACE) inhibitors, angiotensin II receptor antagonists,β-blockers, calcium channel blockers, and diuretics [60] [61]. Any drug has side effects, and various studies have been done about the side effects of antihypertensive drugs [62] [63] [64] [65] [66]. In addition to the side effects previously studied, this study suggests that taking antihypertensive drugs might be a risk factor for ischemic stroke. In other words, ischemic stroke could be a potential side effect, and more careful attention must be paid when prescribing antihypertensive drugs to patients. Furthermore, it was reported that ACE2 is related to the coronavirus infection [67] [68] [69]. These results strongly suggest the careful usage of antihypertensive drugs. Muntner et al. [70] declared that the 2017 ACC/AHA Guideline would increase the use of hypertension drugs and lower the prevalence of CVD events. Like our previous studies [71] [72] [73], this study does not support the 2017 ACC/AHA Guideline or Muntner’s statement.
6. Conclusions
In this study, I analyzed the risk factors for ischemic strokes. Ischemic stroke is one of the most serious diseases in the world. Among the nonmodifiable factors, age, gender and a history of cerebrovascular diseases were important risk factors, as found in previous studies. The history of cerebrovascular diseases is considered to be an especially important factor. In the case of ischemic stroke treatments, time is the most critical factor, and it is necessary to inform the medical personnel of a patient’s cerebrovascular disease history as soon as possible to ensure that proper treatments are used. Hence it is essential that not only the patient but also people around the patient, including family members, colleagues, friends and family doctors, share the information.
Among the modifiable factors, recent large weight change, sleeping well, and taking antihypertensive drugs were considered to be important factors. Among these factors, the former two had the expected results; recent large weight change increased and sleeping well decreased ischemic stroke risks. However, taking antihypertensive drugs did not. Taking antihypertensive drugs increased the probability of ischemic stroke, even after considering its effect of reducing blood pressure. This means that ischemic stroke might be a potential side effect of antihypertensive drugs, and it is necessary to take great care when prescribing antihypertensive drugs. Antihypertensive drugs are classified into several types, and their mechanisms for controlling blood pressure are different. It is also necessary to determine the benefits and side effects of each drug more precisely, including their effects on coronavirus infection through ACE2; however, these were not analyzed in this study. For getting more precise conclusions to evaluate different drug types, analyses using a larger dataset with a longer time-range are necessary. These are limitations of this study and subjects to be studied in the future, and the emergency cooperation of doctors, researchers and medical authorities throughout the world is necessary.
Acknowledgements
This study was supported by a Grant-in-Aid for Scientific Research, “Analyses of Medical Checkup Data and Possibility of Controlling Medical Expenses (Grant Number: 17H22509),” from the Japan Society of Science. This study was approved by the Institutional Review Boards of the University of Tokyo (number: KE17-30). The dataset was anonymized at the health insurance societies. The author would like to thank the health insurance societies for their sincere cooperation in providing us the data. The author would also like to thank an anonymous referee for his/her helpful comments and suggestions.