The study
of copper adsorption onto ion exchange resins of anionic type is part of the
gold recovery from ammonia-thiosulfate solutions, where copper is the main
impurity of the system because it acts as a catalyst of gold dissolution
reaction. A study is made of the adsorption and desorption of copper in the
form of the
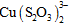
complex in an
ammonia-thiosulfate media on an ion exchange resin, DOWEX 550A, classified as a
strong base, which in its inner structure has a quaternary amine functional
group. In the studied pH range copper adsorption increased with increasing pH,
while the presence of thiosulfate decreased it, the same as the ammonia
content, due to the greater presence of cuprotetramine,
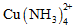
. Elution of the copper
complexes from the resin was more efficient with sulfite than with perchlorate.