An idea on
interfacial equilibrium-potential differences (
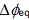
) which are generated for the extraction of
univalent metal picrate (MPic) and divalent ones (MPic
2) by crown
ethers (L) into high-polar diluents was improved. These potentials were
clarified with some experimental extraction-data reported before on the M =
Ag(I), Ca(II), Sr(II) and Ba(II) extraction with 18-crown-6 ether (18C6) and
benzo-18C6 into 1,2-dichloroethane (DCE) and nitrobenzene (NB). Consequently,
it was demonstrated that the

values from the extraction-experimentally obtained
log
KD,Pic ones are in
agreement with or close to those calculated from charge balance equations in
many cases, where the symbol,
KD,Pic,
denotes an individual distribution constant of Pic
﹣ into the DCE or
NB phase. Also, it was experimentally shown that extraction constants based on
the overall extraction equilibria do not virtually contain the

terms in their functional
expressions.