The bis(
N-ethyl-5-methylsalicylaldimine)nickel(II) [Ni(5-me-saletN)
2] complex was synthesized and characterized by elemental analyses, FT-IR, TG-DTA, mass spectrometry and vapour pressure measurement studies. The TG curve of the complex showed a single-step weight loss commencing from 490 K to nil residue at 600 K, without competing fragmentation step. The non-isothermal vaporization activation energy value determined by Coats-Redfern method yielded the value of 93.5 ± 7 kJmol
–1. The dynamic TG run proved the complex to be completely volatile. And the equilibrium vapour pressure
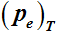
of the complex over the temperature range of 421 - 524 K, determined by the TG-based transpiration technique yielded the value of 94.2 ± 1.2 kJmol
–1 for its standard enthalpy of vaporization
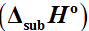
. The entropy of vaporization
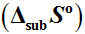
was calculated from the intercept and found to be 249.4 ± 2.6 Jmol
–1•K
–1.