The complexes of bis[
N-alkyl-2-hydroxonapthaldimine]nickel(II) (
N-alkyl = methyl, ethyl, propyl, butyl or pentyl) were synthesized and their volatilization in N
2 atmosphere was demonstrated by the TG-based transpiration technique. The equilibrium vapor pressure of the complexes over a temperature span of 470 - 590 K was determined by adapting a horizontal dual arm single furnace thermoanalyser as a transpiration apparatus. It yielded
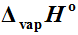
as 153.1 (±1.9), 122.9 (±0.3), 147.6 (±10.7), 151.8 (±10.9) and 114.7 (±5.3) k
·Jmol
–1 respectively. The entropies of vaporization
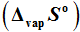
for these complexes as calculated from the intercept of the linear fit expressions were found to be 319.7 (±3.9), 229.9 (±5.8), 317.8 (±17.2), 319.7 (±19.1) and 254.6 (±9.6) Jmo
–1·K
–1 respectively. The non-isothermal vaporization activation energy was determined from Arrhenius and Coats-Redfern methods.