Evaluation of Three Rapid Diagnostic Test Kits in the Diagnosis of Plasmodium Species Infections among Primary School Children in Baringo County, Kenya ()
1. Introduction
Malaria is a major public health problem accounting for 21% of out-patient consultation and 3% - 5% of hospital admissions in Kenya (KMOH [1] [2] ). Children under the age of five years and pregnant women are the most affected with an estimated 34,000 children dying due to malaria each year in Africa [3] . Most of the malaria-related deaths occur in rural areas of the continent due to misdiagnosis resulting in inappropriate or late treatment [4] . In efforts to curb the high malaria-related mortality and morbidity, the Government of Kenya considered malaria intervention measures as a priority investment to achieve its 2030 vision [1] [2] .
Accurate and timely diagnosis accompanied with prompt treatment is an important aspect of malaria management and control [5] . Unfortunately, many treated confirmed cases in Africa are lower compared to other regions of the world [4] . This suggests that majority of treated cases are clinically diagnosed yet this type of diagnosis can be unreliable due to the non-specific nature of signs and symptoms of malaria [5] . It may also lead to indiscriminate use of antimalarial drugs and compromise the required quality care for patients suffering from other diseases displaying symptoms and signs similar to those of malaria [6] [7] [8] . In addition, when transmission rates are low, clinical signs like fevers may not automatically point to Plasmodium spp. infection. Hence parasite-based diagnosis is a vital strategy to avoid inappropriate use of antimalarial drugs [9] .
The WHO recommends that malaria case management be based on parasitological test diagnosis in all cases with exception of young children in endemic areas [6] [10] . Microscopic analysis of malaria parasites and use of rapid diagnostic tests (RDTs) to detect malaria parasite’s antigen in blood, are the two diagnostics most likely to have a greater impact on malaria control today [11] . However, in most remote settings where adequate expert performance of microscopy is needed, this gold standard may be a challenge due to lack of basic microscopy infrastructure [12] . For application of other malaria diagnostic tests such as rapid diagnostic tests to circumvent, the drawbacks are therefore very necessary. Various RDT kits have been in use but their sensitivity and specificity are subject to field evaluation under different endemicity and transmission intensity settings before being recommended for detection of Plasmodium species infections [13] . This is important since their performance may be affected by a number of factors such as, extreme temperatures and high humidity (WHO, 2003), prozone effect [14] and cross-reactivity with human auto-antibodies [15] . In addition, persistence of parasite antigen in blood even after the clearance of malaria parasites by antimalarial drugs often leads to false positive RDT results [11] .
Many malaria RDT kits are commercially available but their performance varies depending on the type of malaria antigen targeted for detection in blood [11] [16] [17] . In the present study, CareStart HRP-2, SD Bioline Ag-Pf and SD Bioline Ag-Pf/Pan RDT kits were evaluated to compare their diagnostic accuracy.
2. Materials and Methods
2.1. Study Site
This study was conducted in three primary schools located within three sub-lo- cations (Barwessa, Kapluk and Keturwo) in the riverine zone of Baringo County. The riverine zone borders the Kerio River, which is to the extreme west of the county. In this area, the soils are poorly drained with a slope of less than 6% making it prone to flooding. Baringo County is located in the former Rift Valley province and lies between 35.602E, 0.541N and 36.277E, 0.723N at altitudes ranging between 870 to 2499 metres above sea level. It is mostly arid or semi- arid, covering an area of 11,075.3 km2 with a total population of 555,561 as per the 2009 Kenya population and housing census (KNBS, 2009). The region has two distinct weather patterns with the temperatures in the southern part ranging between 25˚C during the cold months and 30˚C during the hot months. In the northern parts, temperatures range between 30˚C and 35˚C. The county experiences two rainy seasons; the long rains (March to June) and the short rains (October to November). It receives between 1000 and 1500 mm of rainfall annually in the highlands and about 600 mm in the lowlands.
The most prevalent diseases in this region are malaria and upper respiratory tract infections. Malaria transmission in the region is seasonal and peaks during the rainy season. The current incidence of malaria is about 12% based on outpatient visits to local health facilities (KMOH, 2015).
2.2. Study Design
This was a cross-sectional comparative survey that sought to assess the performance of three RDT kits (CareStart HRP-2, SD Bioline Ag-Pf and SD Bioline Ag-Pf/Pan). Primary school pupils aged 5 to 15 years were examined for infection with Plasmodium spp. using the three kits, with smear microscopy as confirmatory test.
2.3. Sample Size Determination
To determine the performance of RDT kits, sample size was calculated using the formula given by Naing, Winn [18] . Briefly, using the malaria prevalence data of 12%, precision 0.05 and significance level, the minimum number of pupils needed for this study was 162. The sample size was calculated using the formula:
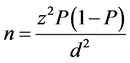
where;
n-Sample size;
z-Confidence level of 95% (standard value of 1.96);
P-Estimated malaria prevalence (12%);
d-Margin of error of 5% (standard value of 0.05).
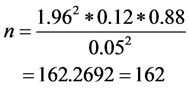
The total number of pupils screened in the first and second surveys was 261 and 300 respectively.
2.4. Sampling Technique
Three primary schools within the riverine zone of the study area were selected based on their proximity to malaria vector breeding habitats. A total of 300 pupils aged between 5 and 15 years were recruited after obtaining informed consent from their parents or guardians.
2.5. Collection and Examination of Blood Samples
A finger prick blood sample (20 µl) was collected from each pupil after a clinical examination and recording of the signs or symptoms. About 5 µl of the blood sample was examined for presence of malaria using SD Bioline Ag-Pf or SD Bioline Ag-Pf/Pan kit and another 5 µl for CareStart HRP-2 kit. The other 10 µl was used to prepare both thin and thick smear for examination under a light microscope.
2.6. Rapid Diagnostic Test
SD Bioline Ag-Pf/Pan (Cat no. 05FK60), SD Bioline Ag-Pf (Cat no. 05FK50C) and CareStart HRP-2 (Cat No. G0140) RDT kits were used to test for the presence of parasite antigen in whole blood, according to the manufacturers’ instructions.
2.7. Microscopy
Thin and thick blood films were prepared and stained using 10% Giemsa stain. The slides were then examined under oil immersion lens (×100). Slide examination was carried out by two microscopists. For positive slides, the number of parasites were counted against 200 leucocytes and quantified as parasite density/µl of blood. Slides were considered negative when no parasite was detected only after examining 100 microscopic fields. Parasite density was determined using the formula below:
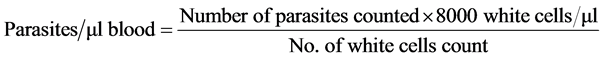
2.8. Statistical Analysis
The diagnostic performance characteristics such as sensitivity, specificity and positive & negative predictive value (PPV & NPV) for each kit were calculated against light microscopy. The true positive (TP), true negative (TN), false positive (FP) and false negative (FN) were used in determination of the characteristics of the kits. The formula used was, TP/TP + FN for sensitivity, TN/TN + FP for specificity, TP/TP + FP for positive predictive value (PPV) and TN/TN + FN for negative predictive value (NPV). The results were interpreted at 95% confidence interval (CI). Since both diagnostic test kits were tested on each pupil (paired data), McNemar’ test was performed to determine if the sensitivities of the two diagnostic test kits were statistically different. Since b + c < 25, McNemar’ test with continuity correction [19] was used.
2.9. Ethical Approval
Ethical approval to conduct the study was obtained from Kenyatta National Hospital and University of Nairobi Ethics and Research Committee (Protocol P70/02/2013). Authority to conduct research was granted by the Director of Health Services, Baringo County. Written informed consent was obtained from the parents or guardians of the pupils and assent was obtained from the pupils themselves before enrolment into the study. Participation was voluntary and confidentiality was maintained. Positive individuals were treated for malaria using Artemether-Lumefantrine (AL) tablets by the study nurse who instructed the pupils to comply with treatment guidelines.
3. Results
During the first survey (June 2015), CareStart HRP-2, and SD Bioline Ag-Pf were evaluated. A total of 261 primary school pupils were tested out of whom 35 (13.4%) tested positive for P. falciparum infections by both RDT kits while microscopic analysis showed that 7 (2.7%) of the blood slides were positive for P. falciparum. The test results of both CareStart HRP-2 and SD Bioline Ag-Pf RDT kits were similar (Table 1).
3.1. Test Performance of SD Bioline Ag-Pf and CareStart HRP-2
The sensitivity and specificity of SD Bioline Ag-Pf and CareStart HRP-2 kits were 85.7% (95% CL: 42.1 - 99.6) and 92.5% (95% CL: 88.6 - 95.4), respectively. The positive and negative predictive values (PPV and NPV) of the two kits for detection of P. falciparum were 24% (95% CL: 9.36 - 4.51) and 99.6% (95% CL: 97.7 - 100), respectively (Table 2).
![]()
Table 1. Comparative performance of RDT (CareStart HRP-2 and SD Bioline Ag-Pf) against light microscopy.
Key: TP = True Positive, FP = False Positive, FN = False Negative, TN = True Negative.
![]()
Table 2. Performance indicators of SD bioline Ag-Pf and CareStart HRP-2 kits (First survey).
3.2. Comparison between CareStart HRP-2 and SD Bioline Ag Pf/Pan
During the second survey (October/November 2015), a total of 300 blood samples from primary school pupils were tested for the presence of Plasmodium species by CareStart HRP-2 and SD Bioline Ag-Pf/Pan. Up to 60 (20%) pupils were diagnosed as positive for malaria. Out of the 300 blood samples, SD Bioline Ag-Pf/Pan detected 60 (20%) samples as positive of which 45 (15%) were positive on the P. falciparum band, 13 (4.3%) positive on both P. falciparum and Pan band while 2 (0.7%) were positive only on Pan band. CareStart HRP-2 diagnosed 50 (16.7%) as positive with 2 invalid test results. In total, 68 (22.7%) of 300 samples were detected as positive for Plasmodium species infections. It was however not possible to tell the two positive cases detected on pan band since the slides prepared from those two samples were read as negative by microscopy (Table 3).
3.3. Performance of SD Bioline Ag-Pf/Pan and CareStart HRP-2
Sensitivity, specificity and predictive values of the SD Bioline Ag-Pf/Pan and CareStart HRP-2 for detection of malaria are shown in Tables 3-6. The overall sensitivity for detection of P. falciparum antigen by the SD Bioline Ag-Pf/Pan was 90% (95% CI: 55.5 - 99.7) while that of CareStart HRP-2was 70% (95% CI: 34.8 - 93.3). The specificity for the SD Bioline Ag-Pf/Pan and CareStart HRP-2 was 82.4% (95% CI: 77.5 - 86.6) and 85.2% (95% CI: 80.6 - 89.1) respectively (Table 4 and Table 5). The study results indicate that the sensitivity of SD Bioline Ag-Pf/Pan kit was slightly higher than that of CareStart HRP-2, while the specificity of CareStart HRP-2 kit was higher than that of SD Bioline Ag-Pf/Pan, though the difference was not significant.
The positive predictive value for detection of P. falciparum antigen by SD Bioline Ag-Pf/Pan and CareStart HRP-2 was 15% (95% CI: 7.1 - 26.6) and 14% (95% CI: 5.82 - 26.7) respectively while the negative predictive value (NPV) for both SD Bioline Ag-Pf/Pan and CareStart HRP-2 kits was 99.6% (95% CI: 97.7 - 100) and 98.8% (95% CI: 96.5 - 99.8), respectively. The SD Bioline Ag-Pf/Pan
![]()
Table 3. Malaria diagnosis by SD Bioline Ag-Pf/ Pan and Care Start HRP-2.
![]()
Table 4. Comparative performance of SD Bioline Ag-Pf/Pan and Care Start HRP-2 against microscopy.
Key: TP = True Positive, FP = False Positive, FN = False Negative, TN = True Negative.
![]()
Table 5. Comparative performance indicators of Care Start HRP-2 and SD Bioline Ag-Pf/ Pan (n = 300).
![]()
Table 6. McNemar’s statistical comparison sensitivity of SD Bioline Ag-Pf/Pan and Care Start HRP-2 kits.
McNemar’s χ2 = 0.5, df = 1, p-value = 0.4795.
had a positive LR of 5.12 (95% CL: 3.7 - 7.07) and a negative LR of 0.121 (95% CL: 0.0189 - 0.78) while that of CareStart HRP-2 was 4.72 (95% CL: 2.89 - 7.71) and a negative LR of 0.352 (95% CL: 0.136 - 0.909).
3.4. McNemar’s Test of Sensitivity
The sensitivity of SD Bioline Ag-Pf/Pan (90%) and that of CareStart HRP-2 (70%) was tested to find out if they were significantly different. Microscopy confirmed 10 out of 300 samples as positive. Out of the 10 confirmed cases, SD Bioline Ag-Pf/Pan detected 9 as positive and 1 as negative while CareStart HRP-2 detected 7 as positive and 3 as negative. The seven samples detected by CareStart HRP-2 as positive were equally detected by SD Bioline Ag-Pf/Pan as shown in Table 6. A chi square test however showed that the sensitivities of both diagnostic kits was not significantly different (χ2 = 0.5, df = 1, p = 0.4795).
3.5. Parasite Density
Parasite density ranged between 160 to 32,800 parasites/µl of blood. The first survey was occasioned by relatively low parasite density of 160 - 3040 compared to second survey (280 - 32,800)/µl of blood. Of the 18 positive blood slides, 8 had parasite density of less than 500/µl, 5 had density below 6050/µl while the remaining 5 blood slides had parasite density ranging between 12,520/µl to 32,800/ µl of blood.
4. Discussion
Parasite-based diagnosis of malaria is important for prompt management. Giemsa microscopy (which detects asexual parasite stages in stained blood smear) and the serological rapid diagnostic tests (RDTs) for detection of parasite antigen represent the two diagnostics most likely to have a significant influence on malaria control [11] . During first survey a total of 261 blood samples were used to determine the performance of CareStart HRP-2 and SD Bioline Ag-Pf kits. Both kits performed well where CareStart HRP-2 and SD Bioline Pf had a sensitivity of 85.7% and specificity of 88.6%. The sensitivity of CareStart HRP-2 was comparable to that reported in other studies such as 89.68% in China- Myanmar [20] , 88.8% in Belgium [21] and 89.1% in Myanmar [22] . The present sensitivity was however slightly higher than that reported from an endemic region in Nigeria at 78.4% [23] . Moreover, the sensitivity of 85.7% for SD Bioline Ag-Pf reported in the present study was similar to the findings from a study conducted in Central African Republic where the SD Bioline Ag-Pf reported a sensitivity of 85.4% [24] .
The high specificity (88.6%) obtained in the present study for diagnosing P. falciparum is indicative of a specific test kit, is important for accurately detecting malaria parasite antigens with low parasite densities which might be easily missed out through microscopy [25] . This is particularly important in remote settings where adequate expert performance of microscopy may be a challenge. The high specificity findings in the present study were comparable to specificity findings reported in endemic areas of Nigeria (97.6%) [23] , 96.21% in Karachi [26] , 96% in Sierra Leone [27] and 94.2% in north-west Ethiopia [28] , but relatively higher than 72% reported in Uganda [29] . The likelihood ratio of a test kit combines both the sensitivity and specificity into a single figure and usually indicates how the test result can reduce the uncertainty of a given diagnosis. For instance, a positive likelihood ratio of >5 and a negative likelihood ratio of <0.2 indicate high chances of a test result of a kit reporting a positive or negative outcome when given a positive screening or negative screening, respectively [30] [31] . Both CareStart and SD Bioline Pf kits had a positive LR of 7.51 and a negative LR of 0.161. This indicated a better performance in malaria diagnosis.
There was relatively low P. falciparum detection by microscopy (13.3%) in the present study compared to those reported in previous studies in Northwest Ethiopia (50.5%) and Wondo Genet in southern Ethiopia (47%) [32] [33] . The current findings further contradict previous studies conducted in China-Myanmar border [20] and North-West Ethiopia [28] which reported more malaria cases detected by microscopy as compared to RDTs. This disparity could however has been occasioned by the difference in the test kits used. Moreover, the low detection of P. falciparum by microscopy in this study could also have been due to the low parasite density as observed during the first survey of the study. The effect of low parasite density in detection of malaria parasites by microscopy was also reported by McManus and Bowles [34] and Snounou, Viriyakosol [35] . Another explanation of low detection of malaria parasites in this study could be due to auto fixation of thick smears due to long storage time before staining occasioned by the expansive nature of the study area, poor road networks and bad terrain, all of which contributed to a time lag between smear preparation in the field, and staining at the laboratory. This could have hindered the identification of parasites in thick smears which usually provide enhanced detection of parasites in blood film for easy quantification, especially in cases of low level of parasitemia [16] .
During the second survey, the performance of SD Bioline Ag-Pf/Pan and CareStart HRP-2 kits were evaluated using 300 blood samples. During this period, both the sensitivity and specificity of CareStart HRP-2 slightly dropped (to 70% and 85.2%, respectively). This could have been due to the few invalid results by CareStart HRP-2, which were however positive by SD Bioline Ag-Pf and SD Bioline Ag-Pf/Pan kits. SD Bioline Ag-Pf/Pan showed a high sensitivity and specificity, similar to other studies which evaluated the performance of the same kit, like a sensitivity of 88.2% reported in the Central African Republic [24] and 97.4% in Greece [36] . The high negative predictive values (about 99%) for SD Bioline Ag-Pf/Pan and CareStart HRP-2 indicate their reliability in ruling out Plasmodium species infection. On the other hand, the kits showed low positive predictive values of about 15% and 14%, meaning that some individuals may be falsely diagnosed as positive, hence confirmation of the positive cases by microscopy before treatment may be necessary, especially where clinical symptoms do not favour malaria diagnosis.
Two samples with high parasite densities were only detected as positive by SD Bioline Ag-Pf/Pan. Failure to detect these two samples could have been due to prozone effect which could have lowered the sensitivity of CareStart HRP-2 kits, by mechanisms earlier proposed Gillet, Mori [37] . Specifically, previous studies conducted to determine prozone effect on HRP-2-and pLDH-based kits reported prozone effects only on HRP-2-based RDT and not on pLDH based RDT [37] . Other studies also reported that sensitivity of HRP-2 based RDT kits were affected by high parasite densities ranging between 10,000 parasite/µl of blood to 100,000 parasite/µl of blood [29] [38] [39] [40] [41] [42] . The present study further reported 0.7% cases of non-P. falciparum plasmodium infections within the riverine zone which were not confirmed through microscopy as they all read negative. Most of the slides which were positive on both P. falciparum and Pan bands on the SD Bioline kits were also positive by microscopy, indicating active infection unlike HRP-2 which persists in blood long after clearance of parasites [5] .
5. Conclusion
The findings of this study show that the performance of the three RDT kits in the diagnosis of P. falciparum infections is relatively adequate and can be used in guiding treatment of febrile illness in remote settings, especially where microscopy may be a challenge. Although SD Bioline Ag-Pf/Pan performed slightly better than CareStart HRP-2, the difference was not significant. Both SD Bioline Ag-Pf and CareStart HRP-2 had similar test results. The three RDT kits can reliably rule out Plasmodium species infection, although they cause false positive diagnosis of P. falciparum.
Acknowledgements
We thank all the children who participated in this study, parents/guardians who gave permission and support for the children’s participation, and teachers for the support during enrollment, screening and treatment. We appreciate the permission from the Ministry of Education, the support of the heads and management of the schools. Appreciation also goes to the Ministry of Health Baringo County for the support, DVBD Marigat laboratory staff and the community members for their support and cooperation throughout the study period. We acknowledge the unequaled dedication of the field team, notably Juliet Jepkosgei, Macrae Mbalanya and Edwin Kibet.
Financial Support
This study received financial assistance from the WHO Special Programme for Research and Training in Tropical Diseases (TDR) through a grant agreement with the International Development Research Centre of Canada (106905-00).
Conflict of Interests
The authors declare that they have no conflict of interests.