Kinetics of Degradation of Eosin Y by One of the Advanced Oxidation Processes (AOPs)—Fenton’s Process ()
1. Introduction
Synthetic dyes are widely used in dyeing, painting, leather making, printing, paper making, cosmetics, photography, and coating [1] [2] . Wastewater especially from dyeing/textile industries contains about 15% of the total dye that is discharged into the nearby water bodies without adequate treatment resulting in water pollution [3] . Water pollution is common in developing countries. One example is Bangladesh. Dyes in wastewaters are non-biodegradable. Phase transfer of pollutants from aqueous system into sludge takes place when attempts are made to remove them by coagulation/flocculation, membrane separation (ultrafiltration, reverse osmosis) or adsorption on activated carbon [4] . To overcome such a problem, advanced oxidation processes (AOPs) were developed to mineralize the organic pollutants. The mineralized components are mainly CO2, H2O and inorganic ions and/or biodegradable compounds [5] . AOPs are considered as promising methods for the treatment of industrial wastewater since they are environmentally friendly.
Eosin Y (EY), a synthetic heterocyclic compound, is a red fluorescent dye in the form of triclinic crystals. All forms of Eosins are bromine derivatives of fluorescein. They are used in dyeing/textiles, ink manufacturing, in coloring cosmetics and gasoline and as a toner. EY is a stable dye and has been used as a catalyst for photocatalytic degradation of some aromatic compounds e.g., arenediazonium salts. The dye shows redox properties with redox potential of 1.1 V (vs. SCE) for the pair EY+/EY* [6] . Wastewater containing EY causes serious environmental problems due to its stability and dark color [6] . Moreover, Muruganandham and Swaminathan [7] reported that the dye, EY, was potentially hazardous to human health.
Among the AOPs, the homogeneous Fenton’s reactions [8] , comprising reactions of ionic iron (Fe2+/Fe3+) and hydrogen peroxide (H2O2), appear to be a promising method to be applied to dye mineralization. This is because of its simple operation, fast initial rate of reaction, low toxicity and it is environment friendly. However, the formation of iron sludge and its effectiveness at acidic pH are likely to be the barrier of its wide applications. AOPs generate hydroxyl radical which is the most reactive oxidizing species in water treatment, with an oxidation potential between 2.8 V (pH = 0) and 1.95 V (pH = 14) vs. SCE (saturated calomel electrode) [9] . The hydroxyl radical is very non-selective in nature and rapidly reacts with numerous organic species with relatively higher rate constants (108 - 1010 M−1∙s−1) resulting in formation of mineralized products like, CO2, H2O and inorganic ions and/or biodegradable compounds [10] [11] .
In the present study, Eosin Y, a dye widely used in many industries, has been taken as a model compound. The homogeneous Fenton’s process has been used to investigate the effects of some kinetic parameters, such as concentrations of H2O2, Fe(II), EY and solution pH on its initial rates of its degradation in aqueous system. The dye is degraded in the dark but the rate of degradation and extent of degradation are enhanced in the presence of UV light.
2. Experimental
2.1. Materials
Reagent grade (RG) Eosin Y (EY) was purchased from the local market and used without further purification. Analar grade (AR) Mohr’s salt, FeSO4∙(NH4)2SO4∙6H2O, hydrogen peroxide (RG), sodium hydroxide (RG), sulfuric acid (RG), oxalic acid (AR), potassium permanganate (RG), potassium nitrate (RG), glacial acetic acid (RG), sodium acetate (RG), and 1,10-phenanthroline were purchased from BDH. Deionized water was used throughout the experiment.
2.2. Experimental Procedures
2.2.1. Preparation of Dye Solution
2.58 × 10−3 M stock solution of EY was made by dissolving 0.42 g dye in a 250 mL volumetric flask with deionized water. The flask was then wrapped with aluminum foil and stored in the dark. Concentrations of the samples of the EY solutions during experiments were determined by measuring the absorbance at 517 nm (λmax of EY) using its molar absorption coefficient (εEY = 5.46 × 104 L∙mol−1∙cm−1 at 30˚C). To calculate the absorption coefficient of EY at 517 nm, five different concentrations of EY were prepared in acetate buffer (pH 4.60) and their absorbance was monitored at 517 nm. The obtained absorbance was plotted against their respective concentrations that showed a straight line which passed through the origin (y = mx, m stands for slope). Beer’s law was obeyed over about 2 orders of magnitude of EY concentration. The absorption coefficient of EY was then calculated from the slope of the straight line by using A = εcl equation. (where ε stands for absorption coefficient, A for absorbance, c for concentration and l for cell path length that is 1). Concentration of the EY stock solution was 2.58 × 10−3 M.
2.2.2. Preparation of Fe(II), H2O2 and Acetate Buffer Solutions
A stock solution of Fe(II) was prepared from requisite amount of Mohr’s salt in a 250 mL volumetric flask by using deionized water containing sufficient amount of sulfuric acid. The permanganate solution was standardized by standard oxalic acid solution. A stock solution of H2O2 solution was prepared through dilution from its 28% solution in a 250 mL volumetric flask and then standardized by the standard permanganate solution. Acetate buffer solution was prepared through mixing of separately prepared acetic acid and sodium acetate solutions. Dilute sulfuric acid or sodium hydroxide solution was added to the buffer solution to adjust the solution pH. Spectrophotometric analysis of the Mohr’s salt stock solution, using 1,10-phenanthroline in acetate buffer (pH 4.60) at 510 nm (λmax of Fe(II)-phenanthroline complex), verified the concentration of Fe(II) in the solution. Here absorption coefficient of the Fe(II)-phenanthroline complex was calculated from the slope of the straight line of absorbance vs. concentration of Fe(II)/1,10-phenanthroline plot by using A = εcl equation. The calculated absorption coefficient (ε) value was found to be 1.11 × 104 L∙mol−1∙cm−1 at 30˚C which nicely satisfies the literature value, 1.10 × 104 L∙mol−1∙cm−1 [12] .
2.2.3. Degradation of EY by Fenton’s Process
A typical experiment was carried out in the following way: 40 mL reaction mixture containing dye solution, acetate buffer solution of known pH and Fe(II) solution was taken in a flask and kept it in a water bath (30˚C). The pH of the mixture was measured at 30˚C by using a pH meter (Orion, Japan). The reaction was started by adding 10 mL H2O2 solution which was also kept at 30˚C. The well agitated reaction mixture was quickly taken into 1-cm quartz cell (Sigma Aldrich) which was kept in the thermostat compartment of the spectrophotometer (Shimadzu UV-160A, Japan). The absorbance of the solution was measured at 517 nm at different time intervals e.g., 1, 2, 3, 5 min.
The degradation of EY by photo-Fenton’s process was carried out in the presence of UV light in a specially designed light house.
2.3. Determination of Initial Rates
Pseudo first-order kinetics provides rather accurate rate data than generally realized i.e. second-order kinetics [13] [14] . Concentration of Eosin Y (EY) was measured at different time intervals e.g., 1, 2, 3, 5 min and the pseudo first-order rate constant (k¢, min−1) was obtained from the slope of Ln A vs. time (min) plot. The initial rates were calculated from the observed pseudo first order rate constants, according to the following Equation (1) and Equation (2):
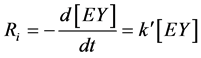 (1)
(1)
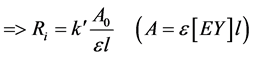 (2)
(2)
where, Ri = initial rate (mol∙L−1∙min−1),
Ao = absorbance at t = 0,
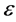 = molar absorption coefficient (L∙mol−1∙cm−1),
= molar absorption coefficient (L∙mol−1∙cm−1),
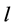 = cell path length (cm) = 1 cm.
= cell path length (cm) = 1 cm.
2.4. Determination of % Degradation
For each set of experiment, the initial absorbance of desired EY concentration was measured at 517 nm, in the presence of all the reagents except H2O2 where aqueous solution of all the reagents except EY, was as reference. After that a new solution of the desired EY concentration was prepared with all the reagents and reaction started just after addition of H2O2. According to the present experimental conditions, the reaction time was 30 min. After 30 min, the absorbance was measured with the same aqueous reagents solution as a reference. In each experiment, the % degradation was calculated according to the following Equation (3):
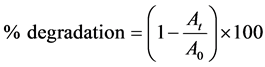 (3)
(3)
Here, Ao = absorbance at time, t = 0 min.
At = absorbance at t = 30 min.
2.5. Statistical Analysis
To validate the experimental results, statistical analysis, such as standard deviation was calculated to quantify the amount of variation or dispersion of a set of data values. A low standard deviation value indicates that the data points tend to be close to the mean of the set, while a high value indicates that the data points are spread out over a wider range of values.
Standard deviation was calculated for each set of experiment through carrying out at least three times, and calculated according to the following Equation (4):
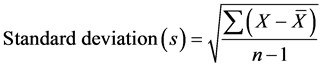 (4)
(4)
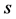 = standard deviation,
= standard deviation,
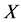 = each value of data-set,
= each value of data-set,
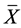 = arithmetic mean of the data,
= arithmetic mean of the data,
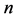 = total number of data points.
= total number of data points.
3. Results and Discussion
3.1. General
Prior to discuss the effects of some kinetic parameters on the initial rates of degradation of Eosin Y by Fenton’s process, it is desirable to focus the characteristics of the dye, EY, on aqueous solution. Eosin Y is an anionic azo dye. It is available as Na-salt (Figure 1). Its spectrum in aqueous solution shows four peaks at 517 nm, 342 nm, 301 nm and 254 nm with molar absorption coefficients 5.46 × 104 L∙mol−1∙cm−1, 0.40 × 104 L∙mol−1∙cm−1, 1.03 × 104 L∙mol−1∙cm−1 and 2.20 × 104 L∙mol−1∙cm−1 respectively (Figure 2). The peak at 517 nm is the most intense and this peak was taken as the λmax, at which degradation (decrease in absorbance) of EY was studied.
The conjugation effect in EY, because of the polycyclic aromatic chromophore, produces the most intense peak at 517 nm. Its peak at 301 nm arises due to the presence of the carbonyl group (-C=O) in the aromatic ring. The relatively weak peak at 342 nm is ascribed to the presence of the carboxylic group in the aromatic rings. The peak at 254 nm arises is due to the presence of benzene ring in the structure of EY. As abovementioned, the presence of the polycyclic aromatic rings is responsible for the most intense peak of EY at 517 nm which is visible range. Therefore, disappearance of color of the EY indicates the degradation of the polycyclic aromatic rings resulting in formation of smaller components and ions, such as CO2, Br−, Na+ etc.
In normal solution (pH ~ 6) EY remains mostly as B (EY2−), as the pH decreases the species C (HEY−) is formed and on the further decrease the species D (H2EY) will be formed as shown in Equation (5) and Figure 3.
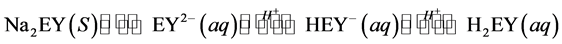 (5)
(5)
![]()
Figure 2. The Spectrum of Eosin Y in aqueous solution at pH 4.60 (Ref.: Aqueous acetate buffer).
The situation is shown below:
In this study, EY has been used to represent any of the forms of EY (Figure 3) although the predominant species at the experimental pH (3.78) is form C. As shown in Figure 4, the absorbance of EY increases with increasing of its solution pH. This is because of the enhancing in conjugation of the aromatic rings of EY structure through formation of carboxylate as well as hydroxylate anions at higher pH that favors the excitation (Equation (5) and Figure 3). However, protonation of the EY2− (D) at low pH (Figure 4) reduces the conjugation which needs higher energy for excitation.
A mixture of H2O2 and Fe(II) ions in the aqueous solution is known as Fenton’s reagent. It has been experimentally proven that Fenton’s reagent generates hydroxyl free radicals (•OH) which attack organic molecules non-selectively resulting in mineralization of the parent molecules [15] . Reactivity of Fenton’s reagent mainly depends on the
![]()
Figure 3. The probable species of EY in aqueous solution at different pH.
![]()
Figure 4. Effect of pH on the absorbance, at λmax = 517 nm, of Eosin Y solution.
rate of formation of reactive species, for example, •OH but irrespective of the EY species.
In our previous studies [16] , we observed no significant difference between % decolorization and % degradation of Ponceau S (PS) by using ZnO-mediated photodegradation through measuring disappearance of PS color and TOC (total organic carbon). Of course, decolorization of organic dyes is faster than their photodegradation. Obviously, the photodecolorization of organic dyes is related to their degradation. Accordingly, studies on photodecolorization gave a measure of the photodegradation of the dyes. Rate of degradation is only depends on the rate of formation of hydroxyl free radicals which depends on the kinetic parameters, for example, solution pH, concentrations of H2O2, Fe(II) and EY. Therefore, it has considered that the disappearance of color (decolorization) of EY solution is proportional to its degradation. According to the present experimental results, the effective solution pH for significant degradation of EY would be 2.74.
3.2. Factors Affecting the Initial Rates of Degradation of EY by Fenton’s Process
3.2.1. Effect of pH on Initial Rate of Degradation of EY
Hydrogen peroxide undergoes auto decomposition at low pH [15] . On the other hand, Fe(III) form hydroxide species through hydrolysis reaction from pH ~ 2.8 resulting in removing of kinetically important Fe species [17] . All these contribute to the lowering of initial rate with rise in pH of the solution (Figure 5). Thus the degradation of EY by Fe(II) + H2O2 could be studied only in a narrow range of hydrogen ion concentration. The decrease in initial rate with pH is typical for a Fenton’s process (Figure 5) [18] .
The minimum decrease in the rate is around pH 4, above which it increases with pH. This can be explained by considering the values of the slopes at pH 4 and 4.5 (Figure 5). The decrease in the initial rate has been attributed to the formation of colloidal ferric species that decompose H2O2 [19] [20] . On the other hand, if the pH is too low, •OH radicals are destroyed according to the Equation (6):
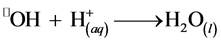 (6)
(6)
3.2.2. Effect of H2O2 Concentration on Initial Rate of Degradation of EY
H2O2 generates hydroxyl free radical (•OH) in the presence of Fe(II) ion. Accordingly,
![]()
Figure 5. Influence of pH on the initial rate of degradation of EY. [EY]: 2.58 × 10−5 M, [Fe(II)]: 2.00 × 10−4 M, [H2O2]: 7.90 × 10−3 M, Temperature: 30˚C.
the concentration of hydroxyl radical (•OH) is expected to be proportional to the concentration of hydrogen peroxide.
At a constant temperature and for a fixed amount of EY and Fe(II), and at a fixed pH, the initial rate of degradation of EY (Figure 6) increases with the increase in H2O2 concentration. However, above a certain concentration of H2O2, ~ 10.00 × 10−3 M, the initial rate remains almost unaffected with further increase in H2O2 concentration at pH 3.78, [EY]: 2.58 × 10−5 M and [Fe(II)]: 2.00 × 10−4 M. The degradation of EY also increases with the increase in H2O2 concentration, but virtually becomes independent of its concentration under these conditions (Table 1).
In Fenton’s process, hydrogen peroxide generates hydroxyl free radical (•OH) through reaction with Fe(II), however, at high H2O2 concentration, hydroxyl radical efficiently reacts with H2O2 and produces hydrogen dioxide radical (HO2•), also •OH radicals generated at high concentration react with HO2• or dimerize to H2O2. It is obvious that HO2• radicals are less reactive than •OH radicals, therefore leading to negligible contribution in degradation of the dye molecules [18] [21] .
3.2.3. Effect of Fe(II) Ion Concentration on Initial Rate of Degradation of EY
The formation of hydroxyl radical (•OH) is also directly proportional to the concentration of Fe(II) ion. Under constant temperature and for fixed concentrations of EY, H2O2 and at a constant solution pH, the initial rate of degradation of EY increases with the increase in concentration of Fe(II) (Figure 7).
The pattern of this increase appears to be unaffected by H2O2 concentration until the H2O2 concentration is more than ~16 × 10−3 M. Three folds increase in H2O2 concentration seems to initiate another reaction path in which the hydroxyl radical (•OH) may react with H2O2 that can be shown by the Equation (7):
 (7)
(7)
![]()
Figure 6. Influence of H2O2 concentration on the initial rate of degradation of EY. [EY]: 2.58 × 10−5 M, [Fe(II)]: 2.00 × 10−4 M, pH: 3.78, Temperature: 30˚C.
![]()
Table 1. Initial rate of degradation of EY by Fenton’s process for reaction time 30 min (Temperature: 30˚C).
The generated •O2H radicals react with EY, but at a slow rate. The ultimate degradation of EY does not change significantly even if Fe(II) concentration is raised to 4.00 × 10−4 M at pH 3.78 and at higher H2O2 concentration (Table 1).
3.2.4. Effect of EY Concentration on Initial Rate of Degradation of EY
The variation of EY concentration (Figure 8) was investigated using Fenton’s process at 7.90 × 10−3 M and 15.8 × 10−3 M H2O2 and photo-Fenton’s process at 7.90 × 10−3 M H2O2. The kinetic parameters, such as concentrations of EY, H2O2 and pH, for the
![]()
Figure 7. Influence of Fe(II) concentration on the initial rate of degradation of EY. [EY]: 2.58 × 10−5 M, pH: 3.78, Temperature: 30˚C.
![]()
Figure 8. Influence of EY concentration on the initial rate of degradation of EY. [Fe(II)]: 2.00 × 10−4, pH: 3.78, Temperature: 30˚C.
Fenton and photo-Fenton’s processes were the same only an ultraviolet light source (UVA) was used in the photo-Fenton’s process.
As shown from Figure 8, H2O2 concentration has the major influence on the initial rate of degradation of EY at definite concentrations of Fe(II) and EY, and at a fixed solution pH. If the H2O2 concentration is below the optimum value, in the present case when [Fe(II)]: 2.00 × 10−4 M and [H2O2]: 7.90 × 10−3 M, lower EY concentration facilitates the following reaction as shown in Equation (8):
![]() (8)
(8)
However when the dye concentration is >2.00 × 10−5 M, the expected reaction, i.e. the degradation of dye starts becoming favorable. The use of UVA (350 - 400 nm light) is responsible to change the situation, that is, increases the initial rate with the increase of EY concentration. This is because the UV light facilitates, primarily, the excitation of some dye molecules resulting in formation of additional •OH radicals. Moreover some dye molecules, however insignificant, may be directly degraded through the generation of dye molecular radicals by the UV light. All these enhance the initial rate of UV-assisted degradation of EY.
It is to be noted that UVA play a significant role on degradation of EY at [H2O2]: 7.90 × 10−3 M, [Fe(II)]: 2.00 × 10−4 M and at pH 3.78 (Table 2). The degradation was observed about 74% with 1.24 × 10−5 M EY concentration within 30 min. Further increase in EY concentration appears to decrease the degradation (Table 2). One of the important reasons for this is the increase of inner filter effect by the increased numbers of EY species in solution. This diminishes the UVA photons that usually enhanced the concentration of •OH radicals through excitation of the EY species [22] .
3.2.5. Effect of H2O2 Concentration on Initial Rate of UV Light-Assisted Degradation of EY
As the UV light can excite dyes, an additional route to formation of (•OH) hydroxyl radical is created [23] [24] .
In a previous study [25] , it was found that UVB (λ < 350 nm) decolorized EY to some extent. In the present case, EY did not seem to undergo noticeable degradation with UVA up to 30 minutes. However when H2O2 at a relatively high concentration was used, there was noticeable degradation of EY in the absence of Fe(II) at pH ~ 6. The variation of initial rate with H2O2 concentration is linear as shown in Figure 9. The intercept with the best fit plot (r2 = 0.9980) (Figure 9) clearly indicates some degradation of EY by UVA is occurred but not detectable within 30 minutes by the present experimental technique.
3.3. Mechanism of Degradation of EY
In the aqueous acidic environment, in the presence of an organic substance, here EY and Fe(II) species, H2O2 initiates a set of complex redox reactions [26] [27] [28] [29]
![]()
Table 2. Initial rate of degradation of EY by UVA with or without Fe(II) for reaction time 30 min (Temperature: 30˚C).
![]()
Figure 9. Influence of H2O2 concentration on the initial rate of UV light-assisted degradation of EY. [EY]: 2.58 × 10−5 M, pH: 3.78, Temperature: 30˚C.
[30] . The overall reactions are shown by the following Equation (9) and Equation (10):
![]() (9)
(9)
![]() (10)
(10)
H2O2 reacts with Fe(II) to generate hydroxyl free radical (•OH) and Fe(II) converted into Fe(III) as shown in Equation (9)). Then Fe(III) reacts with H2O to form hydrogen dioxide radical (•O2H) (Equation (10)). The generated •OH radical reacts with the aqueous dye molecules (EY) to form their molecular radicals (•EY) (Equation (11)) and converted into their cations through reaction with Fe(III) species (Equation (12)).
![]() (11)
(11)
![]() (12)
(12)
Numerous competing reactions, involving Fe(II), Fe(III), H2O2, •OH, •O2H radicals derived from the substances, are possible. The following typical reactions may also take place:
![]() (13)
(13)
![]() (14)
(14)
Here the •OH radical further reacts with H2O2 to form •O2H radical (Equation (13)) and the generated •O2H reacts with Fe(III) resulting in diminishing of the reactive oxidizing species (Equation (14)).
In the presence of UVA, the following important processes can be occurred:
![]() (15)
(15)
![]() (16)
(16)
Here H2O2 produces •OH radical under UV irradiation (Equation (15)) and the dye molecules converted into either their molecular radical (•EY) and/or their cations (EY+) (Equation (16)).
Finally, degradation products (e.g., CO2, ![]() ,
,![]() ) are formed through reactions between the •OH radical and/or other oxidizing species and EY/•EY/EY+ as shown in Equation (17)).
) are formed through reactions between the •OH radical and/or other oxidizing species and EY/•EY/EY+ as shown in Equation (17)).
![]() (17)
(17)
EY stands for H2EY, HEY−, EY2−. Fe(II) and Fe(III) are respective free ions and/or any other of these forms.
4. Conclusions
The present work reveals that the initial rate of degradation of Eosin Y (EY) dye depends on the concentrations of H2O2, Fe(II), EY and the solution pH. Degradation of EY is significantly regulated by its solution pH. Most of the EY species in solution remain as negatively charged species at solution pH ~ 6. The pH of EY solution was kept constant by sodium acetate buffer solution. The buffer did not appear to interfere with the normal rate of H2O2 or Fe(II).
Low solution pH (2.74) shows highest initial rate as well as degradation at [H2O2]: 2.37 × 10−4; [Fe(III)]: 2.00 × 10−4; [EY]: 2.58 × 10−5 M, however, both the initial rate and degradation decrease with increasing of solution pH. This is because of removing of kinetically important iron (Fe) species through formation of ferric hydroxide. H2O2 also increases both the initial rate and degradation up to a certain concentration, after that virtually no effect was observed. This is because of scavenging of the hydroxyl radicals by the H2O2 at its higher concentration. Both the initial rate and degradation were significantly enhanced by using of photo-Fenton’s process. Fenton’s process showed the initial rate: 7.35 × 10−7 mol∙L−1∙min−1 and degradation: 27.62% at [H2O2]: 7.90 × 10−3 M; [Fe(II)]: 2.00 × 10−4 M; [EY]: 2.58 × 10−5 M and pH: 3.78 within 30 min reaction time, while photo-Fenton’s process showed the initial rate: 11.51 × 10−7 mol∙L−1∙min−1 and degradation: 54.32% under the same experimental conditions.
The disappearance of color of EY solution and its mineralization to CO2, Br?, Na+ should take place at different rates [31] . High concentration of only H2O2 degrades EY solution significantly by UVA. This point is the fact that degradation of dyes in the textile factory effluents can be achieved simply with the help of H2O2 and sunlight (containing UVA) at relatively low pH e.g., 3.0.
Acknowledgements
The authors acknowledge the Ministry of Science and Technology of the People’s Republic of Bangladesh for financial support to carry out this work under the project “Photoelectrochemical splitting of water into hydrogen using solar light”.