1. Introduction
Watermelon (Citrullus lanatus, family Cucurbitaceae) is a vine-like flowering plant originally from Southern Africa [1] . It is an important vegetable crop in Africa and can adapt to different environmental conditions [2] . Previous investigations on C. lanatus fruit have highlighted its antioxidant activities, anti-inflammatory effects and several essential nutrients [3] [4] . Intake of C. lanatus fruit has been associated with a reduced incidence of many diseases [2] . C. lanatus fruit is a very rich source of vitamins A and C. Other compounds previously isolated from watermelon include cis-3-hexenal, cis,cis-3,6-nonadienal, cis-3-nonenal, cis-6-nonenal, trans-2-nonenal, cis-2-nonenal, trans,cis-2,6-nonadienal, trans,trans-2,4-nonadienal, and trans,trans,cis-2,4,6-nonatrienal [5] .
The therapeutic effects of C. lanatus fruit have been reported and attributed to its antioxidant and certain phytochemical compounds [6] [7] . For instance, beta carotene and lycopene have been established to play a key role in the treatment of cancer and cardiovascular diseases [8] . Despite the widely reported health benefit of C. lanatus fruit, the profiling of its nutritive contents and antioxidant benefits have not been adequately undertaken in West African species. Therefore, the aim of this study is to identify the phytochemical components present in C. lanatus fruit and assess its antioxidant potentials.
2. Materials and Methods
2.1. Materials and Preparation of Watermelon Juice
All the chemicals used in this study were of analytical grade. The chemicals, solvents, BTH, 1,1-diphenyl-1- picrylhydrazyl (DPPH) were purchased from were purchased from Sigma (USA). Fresh watermelon (C. lanatus) fruits were obtained from Bodija Market, Ibadan in May, 2012 and identified by a qualified Taxonomist. The watermelon was washed thoroughly with distilled deionised water, the outer part (pericarp) was peeled off and the inner part without the seeds were homogenised in a blender. 10 g of this blended watermelon were cold extracted with 100 mL of HPLC grade methanol containing 0.1% HCl, the extracts were filtered over Whatman no. 1 filter paper and residues were repeatedly extracted with the same solvent until they were colourless. Approximately 1000 mL juice was obtained after the filtration. The juice was stored at −20˚C until use.
2.2. Antioxidant Assay
2.2.1. DPPH Radicals Scavenging Assay
The free radical scavenging activity of the fruit extract was analysed using the methods of Sun et al. [9] and Shimada et al. [10] with some modifications. BHT was used as a reference compound. Briefly, 3 mL of sample extract of different concentration (0.00 to 2.0 mg/mL) was added to 1 mL of 0.1 mM methanol solution of DPPH. The absorbance at 517 nm was measured after the solution was kept at room temperature for 30 mins in the dark. The DPPH scavenging effect was calculated as follows:
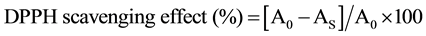
where: A0 = the absorbance of DPPH without sample; AS = the absorbance of sample with DPPH.
2.2.2. Reducing Power
Reducing the power of the various concentrations of watermelon extracts were quantified using the methods of Raza et al. [11] and Yen et al. [12] with some modifications. BHT was used as reference material. Briefly, watermelon extract and BHT were used at different concentrations (0.00 to 2.0 mg/mL). 1.0% of the extract was mixed with phosphate buffer (2.5 mL, 1%). The mixture was incubated at 50˚C for 20 mins, then the reaction was terminated by 2.5 mL TCA solution (0.1%) and the mixture was centrifuged at 3000 rpm for 10 mins. The supernatant (2.5 mL) was mixed with distilled water (2.5 mL) and ferric chloride (0.5 mL, 6 mmol/L), and the absorbance was measured at 700 nm. The increased absorbance of the reaction mixture indicated increased reducing power.
2.2.3. Hydrogen Peroxide Scavenging Activity
Hydrogen peroxide scavenging activity was measured according to the slightly modified method of Zhao et al. [13] . 1 mL each of H2O2 (0.1 mM) and watermelon extract (0 to 2 mg/mL) and standard antioxidant (BHT as control) were mixed separately with 0.1 mL of 3% ammonium molybdate solution and 10 mL of 2 M H2SO4 and 7 mL of 1.8 M KI. The mixture was titrated with 5 mM Na2S2O3 solution until yellow colour disappeared. The percentage scavenging effect was calculated as:
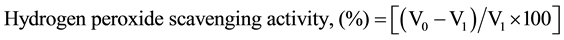
where
V0 = the volume of Na2S2O3 solution used to titrate the control sample in the presence of hydrogen peroxide (without sample extract).
V1 = the volume of the titre value of Na2S2O3 solution used for the extract plus standard antioxidant (BHT as control) mixture.
2.3. Phytochemical Screening, Proximate Analysis and Determination of Beta-Carotene and Lycopene
Chemical tests were carried out on the extract using the standard procedure to identify the constituents as described by Sofowora [13] and Trease and Evans [14] . After milling the samples to uniform size, they were analysed for moisture, protein, fat, ash, fibre and nitrogen free extract by the methods of AOAC [15] . Lycopene and beta-carotene were determined using the methods of Charoensiri et al. [8] .
2.4. Quantitative Determination of Phytochemical
2.4.1. Quantitative Determination of Tannin
Exactly 0.20 mL of sample was added to 20 mL of 50% methanol and placed in a water bath at 77˚C - 80˚C for 1 hr and shaken. The extract was quantitatively filtered using a double layered Whatman No.1 filter paper and 20 mL of distilled water, 2.5 mL Folin-Denis reagent and 10 mL 17% Na2CO3 were added and mixed. The mixture was allowed to stand for 20 min. The bluish-green colour developed at the end of the treatment on the range 0 - 10 ppm. The absorbance of the tannic acid standard solutions as well as samples was read after colour development on a spectronic 21D spectrophotometer at a wavelength of 760 nm. Percentage tannin was calculated using the formula:
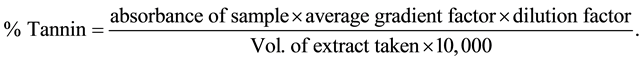
2.4.2. Quantitative Determination of Alkaloids
Two mililitres of sample extract and 1 g magnesium oxide were added to 250 mL of 80% absolute alcohol. The mixture was digested in a boiling water bath for 1.5 h under a reflux air condenser with occasional shaking. The mixture was filtered while hot through a Buchner funnel. The residue was digested for 30 min with 50 mL alcohol and 3 drops of 10% HCl was added. Then 5 mL of zinc acetate solution and 5 mL of potassium ferrocyanide solution was added, and thoroughly mixed to give a homogenous solution. The solution was allowed to stand for a few minutes, filtered through a dry filter paper and 10 mL of filtrate was transferred into a separating funnel and the alkaloids present were extracted vigorously by shaking with five successive portions of chloroform. The residue obtained was dissolved in 10 mL hot distilled water and transferred into a Kjeldahl tube, the digestion was carried out in the presence of 0.2 g sucrose and 10 mL concentrated H2SO4 and 0.02 g selenium for digestion to a colourless solution to determine percentage nitrogen by Kjeldahl distillation method. % Nitrogen is converted to % total alkaloid by multiplying by a factor of 3.26,
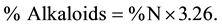
2.4.3. Quantitative Determination of Saponin and Phlobatannin
The spectrometric method of Brunner [16] was used for saponin analysis. For phlobatannin analysis, 0.50 mL of sample was added to 20 mL of 50% methanol and covered with parafilm and placed in a water bath set at 77˚C - 80˚C for 1 hr. The mixture was properly shaken to ensure uniform mixing and later filtered through a Whatman No1 filter paper and 5 mL distilled water was added.1mL of the sample extract was pipetted into a 50 mL volumetric flask. 20 mL water 2.5 mL Folin-Dennis reagent and 10 mL 17% sodium carbonate were added to the solution in the 50 mL flask. This mixture was homogenized thoroughly for 20 mins. 0.5 mg/mL of phlobatannin standard concentrated was prepared from 100 mg/mL phlobatannin stock solution and treated like the sample above. The absorbance of standard solution as well as sample was read on a Spectronic 21D spectrophotometer at a wavelength of 550 nm.
% Phlobatannin was calculated using the formula:
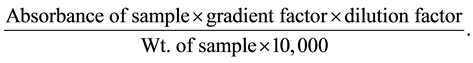
2.4.4. Determination of Total Phenolic and Flavonoids
Total phenolic were determined according to the method of Folin-Ciocalteu described by Makkar [17] , with slight amendments. In brief, 25 μL of crude extract was treated with 250 μL Folin-Ciocalteu reagent for 5 min. The reaction was stopped by adding 750 μL 20% anhydrous sodium carbonate. The volume was made up to 5 mL with distilled water and incubated in the dark at room temperature for 2 h. After incubation, the absorbance was read at 760 nm with a spectrophotometer. The phenolic content was determined from a standard curve of different concentrations of Gallic acid DMSO. The results were expressed as mg/g Gallic acid equivalent (GAE). Flavonoid content of the extracts was determined using the methods of Yadav and Agarwala, [7] , also amended slightly. Crude extracts (100 μL) were dissolved in 300 μL methanol, to which 20 μL 10% aluminium chloride was added. A further 20 μL of 1 M sodium acetate was added to the solution. The resultant solution was made up to 1 mL with distilled water. This was incubated at room temperature for 30 min in a microplate. After incubation, the absorbance was read at 450 nm with a spectrophotometer. Quercetin (10 mM) was used as a standard. The flavonoid content of each extract was expressed as mg/g quercetin equivalent (QE).
2.5. Determination of Lycopene
One gram of fresh water melon was weighed into a 250 ml beaker and crush with a glass rod. 25 ml of HPLC grade acetone was added and shakened for 10 minutes. 25 ml of methanolic NaOH solution was added and a reflux condenser attached. The mixture above was heated in boiling water bath for 1 hour with frequent shaking. The mixture was cooled rapidly and 50 ml of distilled water added. The hydrolysate obtained was transferred into a separating funnel. The solution was extracted thrice with 50 ml of HPLC acetone, 1 g of K2SO4 was added to remove any traces of water. The organic layer was carefully removed into 250 ml beaker and subsequently filtered into a 100 ml volumetric flask and made up to mask with HPLC acetone. Standard solutions of lycopene of range 0 - 50 µg/ml were prepared from 100 ppm stock lycopene solution. The different concentrations of Lycopene standard solutions were treated similarly like sample. The Absorbance or optical Density of samples extracted as well as standard solutions of lycopene were taken on a spectronic 21D Spectrophotometer at a wavelength of 340 nm.
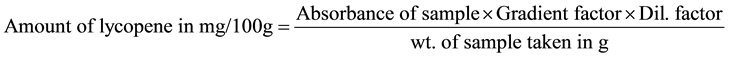
2.6. Determination of Beta-Carotene
Two grams of the water melon sample was weighed into a flat button reflux flask and 10 ml of distilled water was added and mixed carefully to form a paste. 25 ml of alcoholic KOH solution was added. The mixture was heated for 1 hr with frequently shaking and 30 ml of water was added. The solution was retracted three times with 25 ml quantities of chloroform. 2 g anhydrous Na2SO4 was added to the extract to remove any traces of water, the mixture was then filtered into 100 ml volumetric flask and made up to mark with chloroform. Standard solution of β-carotene was made by dissolving 0.003 g of standard β-carotene in 100 ml of chloroform. Absorbance of sample and standards were read using the spectrophotometer at a wavelength of 328 nm on a spectronic 21D Spectrophotometer.
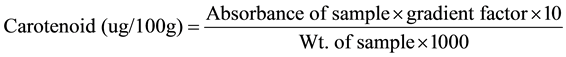
2.7. Statistical Analysis
Data obtained from this study were analysed using the Statistical Package for Social Sciences (version 18.0). Analysis of variance were used to compare values and were considered significant at p < 0.05.
3. Results
3.1. Phytochemical and Proximate Analysis of C. lanatus
Table 1 showed the result of the quantitative analysis of the phytochemicals in watermelon. C. lanatus fruit extract displayed a very high concentration of alkaloids and phenols, a moderate amount of anthraquinone, tannin and saponin (Table 1). Flavonoids, phlobatannin and glycosides were found to be in trace amount.
Using standard procedures, the proximate composition of the watermelon were determined and presented in Table 2. C. lanatus extract showed very high moisture content and its crude protein, crude fat, crude fibre and ash content were all in traceable amounts. The sugar content was considerably high in comparison with other nutritive contents. Lycopene and β-carotene content of C. lanatus extract were estimated to be 4537.83 and 308.71 µg/100g respectively. The Gross Energy evaluation showed a value of 0.335 Kcal/g (Table 2).
3.2. Antioxidant Evaluation of C. lanatus
Figure 1 and Figure 2 showed the DPPH and hydrogen peroxide scavenging activities of C. lanatus extracts and BHT (positive control). C. lanatus extracts exhibited significant (p < 0.05) radical scavenging activity. The
![]()
Table 1. Quantitative analysis of phytochemical in C. lanatus fruit extract.
*Values are expressed as mg/100g dry material and means ± SEM.
![]()
Table 2. Proximate, lycopene and β-carotene composition of C. lanatus fruit.
*Values are means ± SEM.
![]()
Figure 1. DPPH radical scavenging activity of BHT and methanolic extract of C. lanatus. IC50 of methanolic extract of watermelon = 0.10 mg/mL; IC50 of BHT = 0.10 mg/mL.
![]()
Figure 2. Hydrogen peroxide scavenging activity of BHT and C. lanatus. IC50 of methanolic extract of watermelon = 0.62 mg/mL; IC50 of BHT = 0.10 mg/mL.
reducing power of aqueous C. lanatus extracts and BTH are presented in Figure 3. BHT showed highest reducing power of 2.03% and the methanolic extract of watermelon exhibited its highest scavenging activity (1.997%) at a concentration of 2.0 mg/mL.
4. Discussion
Previous studies have focused on the antioxidant and phytochemical components of C. lanatus fruit seeds and peels with scanty information on its juicy part [4] . Consumption of the C. lanatus fruit is universally limited to the juicy part of the fruits. Consequently, this study reported the free radical scavenging activity, essential nutritive values and phytochemicals of C. lanatus fruit extract. The essential nutritive values established in this study
![]()
Figure 3. The reducing power of methanolic extract of C. lanatus and BHT.
indicated that C. lanatus fruit may not be depended on as an exclusive source of protein, lipid and carbohydrate. However, the fruit is enormously rich in phytochemicals and exhibited significant antioxidant activities. Previous studies have highlighted the health benefits of these phytochemicals, and quantitative phytochemical analysis of C. lanatus seed and other parts of the fruits revealed the presence of these phytochemicals in appreciable quantity [4] [18] . Phenolic compounds such as gallotannins, condensedtannins and flavonoids are known to inhibit some molecular targets of pro-inflammatory mediators in inflammatory responses. The phytochemicals also act as antioxidants by scavenging free radicals and thereby attenuate the inflammatory process [4] [8] . Various in vitro and in vivo bioactivities such as anticancer, anti-inflammatory, and anti-allergic properties by flavonoids have been reported [11] [19] .
Furthermore, alkaloids are known to have an effect on the central nervous system and some act as a pain killer (such as morphine). The phytochemical screenings of most plants traditionally used to treat malaria have revealed the presence of alkaloid [20] . Similarly, degenerative disorders, such as gouts and rheumatism, have also been traditionally treated with alkaloid-containing plants. For instance, colchicine compounds are well known in treating gouts [6] . In addition, phenolic compounds, such as anthraquinones have been used as purgatives [21] . Anthraquinones which have a moderate concentration in C. lanatus fruit extract are traditionally used to treat stomachache and constipation. Tannins are dietary anti-nutrients that are responsible for the astringent taste of foods and drinks [22] . Saponin has the property of precipitating and coagulating red blood cells. Some of the characteristics of saponins include formation of foams in aqueous solutions, haemolytic activity, cholesterol binding properties [20] and bitterness [23] . The minute amounts of saponin in C. lanatus investigated suggested that the extract is not toxic.
Flavonoids are potent water soluble antioxidants and free radical scavengers which prevent oxidative cell damage and have strong anticancer activity. The presence of flavonoids in the C. lanatus fruit may infer that the plant can offer protect against inflammation, free radical, microbes and tumor formation [19] . Cyanogenic glycosides are compounds that yield glucose, hydrogen cyanide and aldehyde or ketone upon hydrolysis with an acid or enzyme. Dietary exposure to cyanide occurs mainly in the consumption of food stuffs rich in endogenous cyanide in the form of cyanogenic glycosides. This cyanide could be lethal as it intercalates with cytochrome oxidase for aerobic function [24] . Only free cyanide (CN−) is toxic and if hydrolysis does not occur, the glycoside remains stable and the food using this product becomes safe. The values (0.02 ± 0.001 mg/kg) obtained in this study is below the lethal dose (0.5 - 3.5 mg/kg) for man.
5. Conclusion
Furthermore, in this study, watermelon showed a very high scavenging activity for DPPH and hydrogen peroxide and the activities were comparable with the known standard antioxidant, BHT. This study, therefore, showed that the methanol extract of C. lanatus fruit exhibited substantial free radical scavenging activities. This suggests that the fruit is an important source of natural antioxidant and this potential could be attributed to its flavonoids contents, lycopene and beta-carotene quantified in this study. Consequently, this property makes C. lanatus fruit an interesting target as a good source of antioxidants to prevent or ameliorate diseases whose pathogenesis involves oxidative stress. Therefore, the strong antioxidant properties and the presence of phytochemicals in C. lanatus fruit may justify its popular consumption and usage in herbal medicine. Hence, further research on the bioactivity of its active ingredients could provide more information about the mechanism of action of its various therapeutic values.
NOTES
*Corresponding author.