1. Introduction
Kaolin is a fine clay mineral cream to dark brown, colored by iron oxides/hydroxides (and/or rutile/anatase). Its major constituent is kaolinite (Al2O3∙2SiO2∙2H2O), a hydrous aluminum silicate with a single silica tetra hedral layer linked through oxygen atoms to a single alumina octa hedral layer.
The common ancillary minerals occurring with kaolin include parent rocks like Feldspar and mica, quartz, ferruginous, titanoferrous, and carbonaceous materials [1] [2] . Others include illite, montmorillonite, ilmenite, anatase, haematite, bauxite, zircon, rutile, kyanite, silimanite, graphite, attapulgite and halloysite [3] - [5] .
The most deleterious impurities in kaolin are iron minerals which impart color to the white kaolin. Iron exists as oxides, hydroxides, oxy hydroxide, sulphides and carbonates along with iron stained quartz/anatase and mica in kaolin [2] . Kaolin essentially finds applications in porcelain, nuclear waste treatment, pottery, paper, pigment and filler manufacturing [6] - [8] as well as for catalysts and adsorbent production [9] - [11] .
Extensive research has been carried out on the nature of iron impurities present in kaolin, which leads to the conclusion that iron is present as a part of the kaolinite or ancillary mineral (mica or titania) structure, which can be termed as “structural iron” or as independent iron minerals such as oxides, hydroxides, oxy-hydroxides, sulphides and carbonates, which can be termed as “free iron” [12] .
Beneficiation of kaolin removes deleterious mineral phases and improves the critical properties of the product clay (such as chemical composition, particle size distribution, and brightness) destined for different uses. Size classification using a set of hydrocyclones leads to enrichment of finer clay fractions and the removal of iron and titanium minerals in coarse size ranges [13] . Wet or dry beneficiation process can be used.
The kaolin wet beneficiation process consists mainly of degritting fractionation by centrifuge, high gradient magnetic separation, selective flocculation, chemical bleaching, filtering and drying [14] . Chlorination may also be used for iron and titanium removal [15] . To improve kaolin whiteness, the procedure of beneficiation has to take into account the geological origin of the sample and most of all the nature of its iron impurities [1] .
The aim of the present study is to characterize kaolin from relatively unknown town in southwest Nigeria that has been known for quality ceramics production for ages focusing on the material characterization to identify the crystalline product and compares its properties to those of standards. X-ray diffraction analysis (XRD) is a traditional tool for mineral identification; FT-IR is used to identify the nature of iron present (free or structural), crystal defects, oxidation state of iron and other functional groups present. Transmission electron microscopy (TEM) pictures give the morphology and size of the particles.
2. Material and Methods
2.1. Material
The clay used was mined from Erusu village, near the ancient city of Ajowa Akoko in Akoko Northwest Local Government Area of Ondo state, southwest Nigeria. To avoid contamination from other sources de-ionized water was used for the present study.
2.2. Beneficiation of Clay
The removal of somatic impurities from raw clay was first done by physical separation of dirt, followed by the wet/soaking process according to the procedures reported by Aroke et al. [16] . The powdered bulk sample was soaked in de-ionized water for 48 h (two days). The slurry was plunged and screened through a 65 µm mesh sieve and then allowed to settle; the water was siphoned off and clay was dried for further use.
2.3. Characterization Method
2.3.1. Determination of Cation Exchange Capacity (CEC) with Ammonium Acetate
The sample was prepared, distilled and finally titrated as describe elsewhere [17] . The sample was first ammonium exchanged, and then the ammonium ions in the supernatant were deprotonated into ammonia with sodium hydroxide solution and concentration determined by distillation into a known amount of acid and back titrated (Kjeldahl-method).
2.3.2. BET Surface Area and BJH Pore Size/Volume
Measuring the number of N2 molecules adsorbed at monolayer coverage, gives information needed for calculating the surface areas, which was calculated by the instrument. 0.3 g approx. of the sample was weigh and loaded in to the BET glass sample tube, the weight of the tube before and after loading was recorded. The samples were degassed at 473 K for 3 h by connecting the tube to micromeritics flow prep 060 linked with Nitrogen gas to remove physically adsorbed water molecules. Degassed sample was reweighed and the analysis was carried out in micromeritics Tristar 3000 V4.02 under liquid nitrogen temperature where BET surface area and pore volume/size of the samples were automatically calculated by the instrument using N2 isotherm and the results were recorded on the computer attached to the instrument.
2.3.3. X-Ray Diffraction (XRD)
Qualitative and quantitative determination of the nature of the phases and the amount of the phases that is present in the sample were determined by Panalytical X’Pert Pro diffractometer, employing Cu Kα monochromatic radiation. All the patterns were collected at room temperature with steps of 0.02˚ using a range of 5˚ - 80˚. The measurements were taken at room temperature (298 K), with scan rate of 2˚ min−1 and 0.02 steps and the patterns were recorded by the Broker-D8software.
2.3.4. Laser Raman Spectroscopy
Raman spectroscopy was used because its detection limits are below the 4 nm (approx.) cut-off of crystallite size for XRD and its ability to show bands that cannot be detected using FTIR. The Raman spectra were recorded with Renishaw spectrometer equipped with invia Raman microscope RE 02, 514 nm laser was employed as the exciting source and the measuring parameter were set as follow; accumulation 10, exposure time 20 minutes and laser power between 75%.
2.3.5. Infrared Spectroscopy
The infrared spectra of the sample were collected using attenuated total reflection (ATR) module with a Nicolet model 360 FTIR at 0.5 cm−1 nominal resolution. The spectra were recorded in the region of 4000 - 400 cm−1.
2.3.6. Transmission Electron Microscopic Analysis
Transmission electron microscopy (TEM) images were recorded on a JEOL JEM-2011 high resolution (HR) TEM (JEOL Ltd., Tokyo, Japan). The electrons were produced by a LaB6 crystal and accelerated up to 200 kV. The TEM is capable of producing a beam diameter as small as 0.5 nm, with 0.19 nm resolution. Images were recorded on a Gatan 794 CCD camera (Gatan Inc., Pleasanton, California, USA). A very small amount of the clay was dissolved in isopropanol and loaded into a carbon coated metal grid which gives preferred orientation and allows observation of clay flakes and examination. Various magnifications were used to obtain suitable micrographs of clay minerals.
2.3.7. Thermo-Gravimetric-Mass Spectroscopy (TG-MS)
TGA is a technique in which the weight of a given sample is monitored continuously as a function of time and/or temperature, while under flowing air or nitrogen. TGA was used to probe the decomposition route of the sample and the thermal stability of the clay. The heating rate was set at 283 K/min from 298 to 1273 K in air stream (100 ml/min).
3. Results and Discussion
The CEC of kaolinite minerals range from about 3 - 15 meq/100 g [18] [19] , the low values are probably representative of pure kaolinite and the increase could be due to impurities and pore sizes. Thair and Ollisarapao suggested CEC of 1-10meq/100 also for pure Kaolin [20] . Thus, the calculated CEC of kaolin clay from this work is 8.5 meq/100g; this is within the literature value for kaolinite in any case.
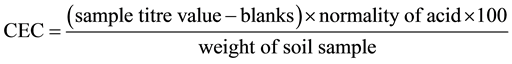
The dispersion forces between the adsorptive molecules and the surface atom or ions of the adsorbing solid are described by the Lennard-Jones potential [21] . Langmuir isotherm is a classical isotherm for a homogeneous flat surface, and most popular of all nonlinear isotherms with monolayer capacity approached at large concentrations by assuming negligible interaction between adsorbed molecules.
BET developed a model describing the adsorption on surfaces describing multi-layers [22]
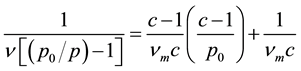
Basically, BET is an extension of the Langmuir treatment to multilayer adsorption on a homogeneous, flat surface. It is useful for gas-solid systems in which condensation is approached. It takes no account of porosity [23] .
The result obtained from N2 adsorption-desorption studies of kaolin clay at liquid nitrogen temperature (77 K) is given in Figure 1 and Figure 2. The result obtained show that kaolin clay is majorly a mesoporous material and the isotherm is that of type IV. The micropore surface area obtained from t-plot is 9.06 m2/g indicating that the material also contain micropore.
The BJH method was originally developed for relatively coarse porous adsorbents having a wide range of pore sizes.
However, the procedure proved to be applicable to almost all types of porous materials.
The distribution of pore volume with respect to pore size is called a pore size distribution. It is generally accepted that the desorption isotherm is more appropriate than the adsorption isotherm for evaluating the pore size distribution of an adsorbent. The surface properties of the material are given in Table 1, the pore size is 15.611 Å and the pore volume is 0.265 cc/g. This value were taken from BJH cumulative desorption in Table 1. The pore size distribution shows the material to be mainly mesoporous as the region within 2 - 50 nm had highest point. This is followed by micropore 0 - 2 nm and macropore > 50 nm. Hence the order is mesoporous > micro pores > macro pores.
The diffraction pattern from xrd is shown in Figure 3, this give kaolinite and quart as the major minerals in our sample. However feldspar, haematite and rutile are also present in minor quantities. The diffraction patterns obtained using ICDD 00-029-1490 as the reference code are 12.22, 20.59, 25.09, 27.95, 35.92, 49.78, 55.11, 59.60, 62.35, 77.03 which corresponds to kaolinite. The other patterns indicated the presence of other types of clay in the tested sample.
![]()
Figure 1. Nitrogen adsorption isotherm for Akoko clay.
![]()
Table 1. Surface properties of Akoko clay.
![]()
Figure 2. Pore size distribution of Akoko clay.
![]()
Figure 3. X-ray diffraction pattern for Akoko clay.
To determine if there are any band in the clay that is Raman active, Raman spectroscopy study was conducted on the sample and it was observed that the material was not Raman active. The spectral obtain is given in Figure 4.
The ir methods are still less widespread for quantitative determination of clay and other minerals. The absorbing bands for most clay minerals and associated minerals are given by farmer (1974) [24] .
Absorption bands of some clay minerals, data Farmer (1974, Gadsden (1975) [24] [25] .
Mineral: Absorption bands (cm−1).
Kaolinite: 3695, 3660, 3625, 1035, 1020, 915.
Halloysite: 3696, 3624, 3414, 1035, 1005, 910.
Montmorillonite: 3635, 3400, 1640, 1130, 1020, 920.
Chlorite: 3586, 3560, 3436, 1004, 980.
![]()
Figure 4. Laser Raman spectrum for Akoko clay.
The spectral can be divided into two regions as shown in Figure 5: 4000 - 1300 cm−1 (functional group region) and 1300 - 400 cm−1 (finger-print region). The frequency assignment approach is adopted for the current investigation [26] [27] .
The bands placed between 3744.64 and 3620.97 cm−1 region corresponds to -OH stretching. The 3620.97 cm−1 frequency band corresponds to the inner layer OH(Al-O―H) stretching which falls close to 3623 and 3622 cm−1 obtained by Burhan and Emin, 2009, and Yleana, 2005 [28] [29] . The absorption band at 1120 cm−1 is assigned to Si-O normal the plane stretching. The bands placed at 1028 - 910.78 cm−1 region corresponds to Si-O planar stretching which agrees closely with 1027 - 1009 cm−1 reported by Burhan and Emin. The frequency vibration 910 cm−1 assigned to OH deformation linked to Fe3+ and Al3+. The IR spectra clearly showed predominance of kaolin in the studied sample and did not exhibit any peak for impurity such as smectite. The spectral region between 800 and 750 cm−1 is very sensitive against the crystallinity and purity of the kaolinite mineral. Pure kaolinite exhibits two peaks 797 and 750 cm−1 in this region and a well crystallized kaolinite exhibits two sharp peaks at 3690 and 3620 cm−1 (Giesel, 1988) [30] .
Transmission electron microscopic analysis (TEM) was used to directly image nanoparticles of the raw clay at scales approaching a single atom and the result is presented in Figure 6. Tem generally can be used for observation of single clay mineral grains. Stacking of layers, thickness of domain and nature of interlayering can be observed, provided that resolution is good enough. From our result, we observed the presence of hexagonal kaolinite particles. The particle size distribution analysis of the raw clay also shows that it’s predominantly fine particles (fraction greater than 2 µm).
Upon heating in air and under inert condition kaolin starts to lose water at approximately 400˚C, and the dehydration approaches completeness at approximately 525˚C [31] . The dehydration depends on the particle size and crystallinity. Loss of absorbed water begins at 100˚C to 200˚C. Loss of structural water (hydroxyls) 450˚C to 500˚C and complete at 600˚C to 750˚C. At 800˚C to 900˚C disintegration of crystal lattice and produces a variety of phases, such as mullite, cristobalite and cordinerite. Figure 7 and Figure 8 represented the TG-MS of clay in Air and Argon respectively. From our results, the sample exhibited a decompositional water loss of 13.2195% and 13.1378% in air and argon respectively. This result is ideal for Kaolinite because its loss in weight from literature is 14% [19] [32] .
4. Conclusion
In the present study, the physic-chemical characterization of Akoko Kaolin was attempted using different analytical tools such as cation exchange capacity, BET surface area and BJH pore size/volume, X-ray diffraction (XRD), laser Raman spectroscopy, infrared spectroscopy, transmission electron microscopic analysis and thermo-gravimetric-mass spectroscopy (TG-MS). The Brunanuer-Emmett-Teller (BET) analysis showed that the kaolin clay was majorly a mesoporous material and the isotherm was of the type iv. The micropore surface area
![]()
Figure 5. Infrared spectra for Akoko kaolin clay.
![]()
Figure 6. Transmission electron microscopic image of Akoko clay.
![]()
Figure 7. Thermo-gravimetric-mass spectral in air for Akoko clay.
![]()
Figure 8. Thermo-gravimetric-mass spectral in Argon for Akoko clay.
obtained from t-plot is 9.06 m2/g indicating that the materials also contain micropore with size and volume of 15.611 Å and 0.265 cc/g respectively. The XRD, IR and TEM analysis confirmed the presence of Kaolin and Quarts as the major constituents of Akoko clay. The result obtained when kaolin was heated in air and under argon atmosphere conditions indicated three decomposition routes, the first steps was due to losses of physically adsorbed water at 100˚C - 200˚C, the second at 450˚C - 500˚C which was due to loss of structural water while the third was at 600˚C - 850˚C which may lead to complete disintegration of kaolin. Finally, Akoko clay exhibited decompositional water loss of 13.23% and 13.14% in air and argon respectively at 1000˚C.
Acknowledgements
The author wants to appreciate the technologists Mr. Adeyemo, Mr. Bamidele and other laboratory staff of the Department of Chemical Sciences, Adekunle Ajasin University Akungba Akoko for their support during the research. Mr. Olatundun Stephen aka Mr. Clay for preparing the sample for this work. Mr. Oloye Femi Francis for his contributions in making this work successful.