Nutritional Yield of African Indigenous Vegetables in Water-Deficient and Water-Sufficient Conditions ()

1. Introduction
Water scarcity in parts of sub-Saharan Africa is spreading due to a decline in annual rainfall levels and overuse of renewable water sources [1] . Increased demand for water from growing populations and the effects of climate change intensify the impact of water scarcity experienced in semi-arid and arid regions of Africa [2] . Domestic water usage, however, is only one factor contributing to declining water levels. The production of industrial and agricultural goods traded internationally also consumes water. Inadequate local, regional and national water supplies directly affect the amount of available drinking water and food. Dry seasons are prolonged as a consequence of decreased water quantity in Africa, widening the already existing “hunger gap” that occurs in the dry months. The Intergovernmental Panel on Climate Change (IPCC) predicts that by the end of the century, Africa as a whole will have an increase of 5% to 8% in dryland and up to 50% reduction in agricultural production in some countries [2] .
Water scarcity is a major threat to food and nutritional security [3] . Regional declines in water availability result in drought, erosion, soil-nutrient deprivation and high salinity [4] . Ultimately, the soil becomes unproductive and the area of arable land is reduced. Adaptation to water shortage is one of the main focuses of climate change adaptation programs in Africa [4] [5] . The aim of such programs is to reduce water wastage and excessive withdrawals that may cause the current food security situation to deteriorate. Food security amid low water availability could be achieved through sustainable water resource management.
Among various adaptation mechanisms, selection and promotion of nutritious food crops with low water requirements for smallholder farmers and home gardens can improve a community’s nutritional and health status. Past and present foreign influences on African cultures have altered traditional diets [6] . However, the usage and cultivation of traditional or indigenous vegetables is still widespread in most African countries [7] -[9] . Indigenous vegetables such as amaranth (Amaranthus sp.), jute mallow (Corchorus olitorius), African nightshade (Solanum scabrum and S. villosum) and Ethiopian kale (Brassica carinata) are commonly consumed by African households [10] [11] . Compared with some widely consumed vegetables such as common cabbage, tomato, and onions, many indigenous vegetables are relatively rich in vitamins, minerals, dietary fiber and protein [12] . Moreover, consumption of leafy indigenous vegetables is more prevalent in poor households as emergency food sources during shortages [9] [13] . Indigenous vegetables are mostly collected from the wild rather than cultivated. Some local leafy vegetables previously studied for susceptibility to drought include African-type amaranth and nightshade [14] -[16] .
Few studies have examined the nutritional yield of these indigenous vegetables under water-limited conditions. Nutritional yield (biomass yield x nutrient content) refers to the amount of nutrient in the edible portion of the total biomass of the vegetable. Low biomass production due to water deficiencies may result in lower nutritional yield, as water is a crucial active transporter of plant nutrients [17] . Plant responses to water-deficit include: decreased leaf area, leaf abscission, root extension, stomata closure and decreased photosynthetic activity [18] . These responses would affect plant nutrition.
Food crops promoted for community climate change adaptation programs should be drought tolerant, and high in nutrient content. The purpose of this study is to assess the nutritional yield of leafy indigenous vegetables in water-deficient and water-sufficient conditions. The experiment was conducted to examine the feasibility of using these vegetables as nutritional alternatives in water scarce regions. It also serves as a pilot study for the cultivation of selected leafy indigenous vegetables by smallholder farmers and household gardens in Africa.
2. Materials and Methods
2.1. Crops
Three vegetable crops (Amaranth, African nightshade and Ethiopian kale) were chosen due to their similar growth rates, and popularity in production and consumption throughout Africa [19] . Two varieties from each of the three vegetable crops (for a total of six entries) were selected for the study based on their African origin, nutritional content and consumer acceptability: amaranth species Amaranthus cruentus var. AC-NL (named A1 in the study) and Amaranthus hypochondriacus var. AH-TL (A2); Ethiopian kale (Brassica carinata) varieties Mbeya Green (K1) and ST3 (K2), and African nightshade species Solanum scabrum Line BG 16, bitter type (N1) and Solanum villosum var. SV, sweet type (N2) (Table 1). The seeds for the experiment were obtained from AVRDC-The World Vegetable Center’s regional office in Arusha, Tanzania.
2.2. Growing Conditions
The experiment was conducted in a glasshouse at AVRDC-The World Vegetable Center, located in Shanhua,

Table 1. Common name and scientific name of six African indigenous vegetable entries.
Taiwan (latitude 23˚8'3'' N, longitude 120˚17'18'' E). The glasshouse was controlled at a day photoperiod of 13 h and maintained at the relative humidity of 75% with a daytime temperature of 35˚C and night-time temperature of 25˚C. The plants were grown in a sandy loam potting mixture containing soil: rice husk: sand: organic matter in a ratio of 3:1:1:2.
2.3. Experimental Design
Two water treatments, water-sufficient (WS) and water-deficient (WD) were applied to the six vegetable entries. The 12 treatments (2 water treatments × 6 entries) were arranged in a randomized complete block design (RCBD) with 4 blocks. Each treatment included three plants with one plant grown per pot. In addition, three plants per entry were grown for observation of days to wilting, days to recovery from wilting with watering, and days to death without watering. These plants were not included in the plant and nutritional measurements.
Seedlings were sown into 70-cell seedling trays (340 mm × 285 mm × 50 mm). Two seeds were planted per cell and thinned to one plant after appearance of the first true leaf. 20 days after sowing, the seedlings at 4-leaf stage were transplanted into plastic pots (140 mm × 140 mm) with one plant per pot, each containing 3.5 kg of weighed potting mixture. Soil moisture of each pot was measured daily using a portable soil moisture meter (Fujian E-INGINST Electron Co., Ltd., China) on a scale of 1 to 10 (1 - 3, dry; 4 - 7, appropriate water content; 8 - 10, wet).
All the plants were watered to the scale of 8 for the first week after transplanting. Otherwise, plants reached the terminal wilting point approximately 3 days without watering after transplanting. The water treatment started on the 27th day after sowing. The water-sufficient treatments were watered to the scale of 8 once every morning until harvesting day. The water-deficient treatment was watered once when plants showed signs of wilting which was the 37th day after sowing (10th day after treatment). The watering allowed the plants to recover from wilting. The water treatment is illustrated in Table 2.
Amaranth, Ethiopian kale, and nightshade treatments in all blocks were harvested at 40 days, 43 days, and 45 days after sowing, respectively, when plants reached commercial maturity. Plants within plots were bulked at harvest, measured for plant traits of interest, and prepared for analyses of nutritional traits.
2.4. Plant Measurements
Leaves, roots and stems were separated from the three plants in each plot. Fresh weight was determined and dry weight was measured after drying in an oven for 48 h at 85˚C. The weight of the edible portion is a combined weight of tender leaves and young shoots.
2.5. Sample Preparation for Nutritional Analyses
The edible portion (tender leaves and young shoots) was weighed and divided in half for oven drying to determine dry matter and mineral contents. The other half was divided into several 20 g samples for storage at −70˚C to measure antioxidant activity (AOA), ascorbate content and for freeze-drying to measure carotenoids and tocopherols content. Nutritional yield was calculated as follows: Nutritional yield (mg∙plant−1) = Nutrient content (mg (100 g)−1) × Fresh edible yield (g∙plant−1).

Table 2. Watering plan for water-sufficient and water deficient treatments.
2.6. Dry Matter and Minerals
Oven-dried samples were ground into fine powder with cyclone mill (Pulverisette 14701, FRITSCH, Germany). Dry matter was determined from the weight difference of 0.5 g of fine powder before and after placing in an oven at 135˚C for four hours. The determination of calcium, iron, and zinc contents was performed by ashing procedure, strong acid washing and then detection with atomic absorption spectroscopy [20] .
2.7. Carotenoids
The primary carotenoids in leafy vegetables are neoxanthin, (all-E)-violaxanthin, (all-E)-lutein and (all-E)-β- carotene [21] [22] . Commercial carotenoids were purchased and used for identification and qualifications. Carotene analyses in the laboratory were carried out under red light. Glassware was either brown-colored or protected from light. According to preliminary tests, saponification was not necessary for carotenoid analyses of the three crops. Carotenoid extraction and separation were performed following the procedure of our previous work described in Hanson et al. [23] . Briefly, about 200 mg of freeze dried fine powder was rinsed in 0.8 ml of distilled water followed by 9.2 ml of acetone. The extract was evaporated under a nitrogen air flow and re-dissolved in 2 ml of 1% tetrahydrofuran (THF) in methanol.
Separation and identification of carotenoids was performed using a HPLC system (Waters 2695, Milford, MA, USA) equipped with an auto-sampler, a photodiode array detector (Waters 996) monitoring 210 - 700 nm, Millennium software, and a C30 Column (YMCTM, 3.0 μm, 4.6 mm × 150 mm). The running conditions were set at 30˚C using a gradient at 1.3 ml/min from 0% to 1% THF in methanol at 0 - 15 min, 1% to 25% THF in methanol at 15 - 25 min, 25% to 70% THF in methanol at 25 - 50 min, and the final 100% THF at 50 - 60 min. Identification of sample carotenoids was performed by comparing retention time and light absorption spectra (350 nm - 700 nm) of known standards and co-elution of spiked known standards to samples. The peak areas were calibrated against known amounts of standards. The content of carotenoid standards was measured according to their OD reading and specific extinction coefficient (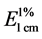 ) in respective solvent.
) in respective solvent.
2.8. Tocopherols
Freeze-dried samples were ground into fine powder by cyclone mill. About 200 - 500 mg powder was used for tocopherol analysis. The extraction and saponification procedures were followed as described in Osuna-García et al. [24] . Separation and quantification were performed using high performance liquid chromatography (HPLC Waters, Midford, MA, USA) equipped with a 717 plus autosampler, 600E solvent delivery system, 2487 detector (read at 294 nm) and a data management system (Empower software). A 250 × 2 mm Phenomenex Prodigy ODS-2 column (5 mm particle size) was used under isocratic conditions at ambient temperatures. The mobile phase was acetonitrile: methanol (85:15, v/v) at a flow rate of 0.4 ml∙min−1. Commercial α-, γ-, δ-tocopherols were used for calibrations. Total tocopherols content is a combination of α-, γ-, δ-tocopherols.
2.9. Ascorbic Acid
Sufficient plant samples for ascorbic acid analysis were available only for amaranth treatments. The determination of total ascorbic acid of frozen sample was carried out as described in our previous work by Hanson et al. [25] on the basis of coupling 2,4-dinitrophenylhydrazine (DNPH) with the ketonic groups of dehydroascorbic acid through the oxidation of ascorbic acid by 2,6-dichlorophenolindophenol (DCPIP) to give a yellow-orange color in acidic conditions [26] .
2.10. Antioxidant Activity
Frozen samples (20 g each bag) were mixed with 80 ml of methanol in water (4:1, v/v). The methanol extracts were measured for antioxidant activity with ABTS 2,2'-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid). The capacity of different components to scavenge the ABTS radical cation was compared with that of trolox in a dose-response curve. The antioxidant activity was expressed as μmole trolox equivalent (μmol TE). The detailed methodology was described in our previous work by Yang et al. [27] .
2.11. Statistical Analysis
The data were subjected to analysis of variance (ANOVA) using the SAS General Linear Model procedure (SAS Institute, Cary, NC, USA). The entry (E) × water (W) interaction mean square tested the effect of the water treatments on the plant response and nutritional values of the different entries. The entries within each crop group were compared. The effect of the water treatment on the entries of the different crop groups (E × W) was assessed.
3. Results and Discussion
The effects of entry, water treatment, and entry x water interaction were tested for plant response and nutritional measurements. Plant measurements included leaf, root and stem weights, and root and stem heights. Nutritional measurements included antioxidant vitamins (carotenoids, tocopherols, ascorbate), antioxidant activity and minerals of edible portion (EP). The p values of the statistical results are presented in Table 3.
3.1. Analysis of Variance
Highly significant differences (p < 0.01) were found in the effects of entry (E) and water (W) treatment for plant weight, plant height and edible yield. The effect of water treatments on the entry (E × W) was significant for stem and leaf weight.
For nutritional values, the results for the content (based on 100 g EP) and yield (based on actual plant EP) showed different patterns (Table 3). All the nutrient yield results showed significant effects of entry and water. When the content was analysed, no significant difference was observed in water effect for most of the nutrients tested including α- and β-carotenes, α- and total tocopherols, iron and zinc.
3.2. Plant Measurements
The plant weight and height measurements showed that the water-deficient treatment limited root, stem and leaf growth (Table 4). Water-deficient treatment seriously affected plant growth by reducing total dry weight biomass between 10% - 60% of total plant dry weight. Among the six entries, A2 lost the most amount of total fresh weight (71.6 g per plant) followed by N1 and K1. Root growth and elongation is a known plant response to water deficit for plant health. The water-deficit condition was severe and the roots systems of the six entries were not able to grow and elongate in response to the drought and maintain plant health. Among the crops, Ethiopian kale had lower root weight and shorter root length than amaranth and nightshade.
The early plant response to water-deficit is to inhibit cell expansion, which results in decreased leaf area to reduce transpiration. Continued water stress causes leaf senescence and abscission. This response is reflected in the reduced leaf area of the crop entries and a decrease in photosynthetic activity. However, specific leaf area, which accounts for total leaf dry weight, measures the crop’s leaf expansion against nutrient conservation [28] . In general, drought tolerant plants tend to have lower specific leaf area values than drought vulnerable plants [18] . The differences among crop groups suggest that the Ethiopian kale group was more susceptible to drought stress than amaranth or African nightshade. Moreover, N1 has a higher leaf water content compared to N2. This will give N2 a competitive advantage under drought conditions as it has lower leaf water content even under controlled conditions.
Root elongation and growth is another plant response to water deficit, which in this experiment did not have a significant effect [29] . Under drought conditions, as the surface soil dries up, the roots should extend to deeper moist soils. A deeper root system will allow plants to extract more available water from the soil [30] . The rootto-shoot ratio should be higher in water-stressed plants as growth is concentrated in roots rather than aboveground biomass [31] . However, the plants in this experiment did not show this response. The root-to-shoot ratio

Table 3. p values for mean squares from the analysis of variance of plant measurements and nutritional measurements of six African indigenous vegetable entries subjected to water-deficient and water-sufficient treatment.
NS, p > 0.05; Cont, nutrient content; Yield, nutrient yield; AOA, antioxidant activity.

Table 4. Plant fresh weight measurements (g per plant) of six African indigenous vegetable entries subjected to water-sufficient (WS) and water-deficient (WD) treatments.
HSD, honestly significant difference.
only showed a significant difference for African nightshade. An explanation is that the water stress was initiated once the plants were established and did not affect the plants early in the critical root growth stages. Another reason may be due to the potted experiment as plants were not planted in field conditions, the limited depth of the pot could have easily dried up the soil, not allowing roots to find and extend to deeper moist soil. Overall, the fresh stem and leaf weights are decreased under water-deficiency for all crop groups. The total dry weight suggests that the amaranth group had the highest dry yield. Furthermore, early flowering is a common plant response to water stress [32] . Early flowering occurred in the amaranth and African nightshade groups under water-deficit, which may have reduced vegetative growth [33] .
3.3. Nutritional Values
The nutritional content and nutritional yield (nutrient content × fresh edible yield) (Tables 5-8) of the vegetables showed different effects in the water-deficient treatment compared with the water-sufficient treatment. The water-deficient condition limited the plant stem, leaf and root growth leading to lower biomass and edible yield, and thus implicated in a lower nutritional yield. However, the accumulation of the phytonutrients including α-carotene, β-carotene, α-tocopherol, ascorbic acid, iron and zinc per 100 gram of edible parts was not significantly affected by the water-deficient treatment (Tables 5-8). Overall, the nutritional values in the yield results demonstrated a decreasing trend in the water-deficient treatment, which is consistent to the decrease in fresh edible weight. In the content results, apart from ascorbic acid and tocopherols, most values show an increasing trend. It is worth noting that the selection of vegetables based on either nutrient content or nutrient yield may result in different choices due to the trends of the content and yield results shown in this study.
The nutritional values of amaranth, African nightshade and Ethiopian kale were affected by water deficits on

Table 5. Carotenoid content (mg per 100 g EP) and yield (mg per plant EP) of six African indigenous vegetable entries subjected to water-sufficient (WS) and water-deficient (WD) treatments.
HSD, honestly significant difference; EP, edible portion.

Table 6. Tocopherol content (mg per 100 g EP) and yield (mg per plant EP) of six African indigenous vegetable entries subjected to water-sufficient (WS) and water-deficient (WD) treatments.
HSD, honestly significant difference; EP, edible portion.

Table 7. Antioxidant activity and ascorbic acid measurements of six African indigenous vegetable entries subjected to water-sufficient (WS) and water-deficient (WD) treatments.
HSD, honestly significant difference; EP, edible portion; -, no data.

Table 8. Mineral content (mg per 100 g EP) and yield ((mg per plant EP) of six African indigenous vegetable entries subjected to water-sufficient (WS) and water-deficient (WD) treatments.
HSD, honestly significant difference; EP, edible portion.
two levels. First, at the content level, where all the values were calculated based on 100 g quantities, most values, with the exception of ascorbic acid, showed an increase in the water-deficient treatment compared to the watersufficient treatment for all the entries. Under drought stress conditions, antioxidants and specific osmolytes accumulate to maintain cell turgor and stabilize cell proteins [34] . AOA values increased in water-deficit conditions in the content results. Carotenoids and tocopherols are fat-soluble compounds while ascorbic acid is watersoluble. Therefore, ascorbic acid values may have decreased at the content level due to the loss of water, its main transport mechanism [35] . Transpiration and other processes of plant water loss during drought may have increased the content of micronutrients and minerals in the plants [36] .
Second, once the content values were recalculated to account for the actual fresh edible weight of each entry, the nutritional values reflected a decrease in overall nutritional yield under water stress. The slight content increases are masked by an overall decline in fresh edible weight in water-deficient entries. Nutritional value is strongly related to crop yield. Although at the content level, the entries showed higher values in drought-stressed conditions, its overall yield is compromised. The actual nutritional yield values have a greater implication for crop selection and food security than their content values.
From the experimental results, A2 (A. hypochondriacus) can be considered to be more drought tolerant than A1 (A. cruentus) and retained higher nutritional values. N2 (S. villosum), however, generally showed higher nutritional values in the water-sufficient treatment, but had higher losses in the water-deficient treatment than N1 (S. scabrum). Therefore, N1 (S. scabrum) is the more nutritious choice for African nightshade. K1 and K2 (B. carinata) had the least drought tolerant among the six vegetables regarding the most severe loss of edible portions and lower nutrient contents and yields. Indigenous vegetables are considered to have stronger water stress adaptation mechanisms than common, commercially available vegetables [14] . Further research could include a comparison of the nutritional yield in indigenous and non-indigenous vegetable food crops under water-deficient conditions.
3.4. Limitations and Future Study
The crop yield was affected by many uncontrollable variables in the glasshouse. Both Ethiopian kale varieties were attacked at seedling stage by whiteflies and diamondback moth larvae. Pest problems early in the growth stage of the crop reflect a reduced yield at maturity. African nightshade was subjected to spider mite infestation approximately 10 days before harvest. The consequences were reduced growth and leaf abscission, which severely reduced the yield.
The experiment was meant as a pilot study to a larger scale, open-field experiment in semi-arid Africa. The glasshouse conditions, such as temperature, daylight period and relative humidity of this pilot study did not fully reflect the situation in Africa. Water scheduling instead of water quantity was used in this experiment to imitate practices of home gardeners or smallholder farmers. Growers will only water their plants when there is water available. It may take many days before water can be collected and used. Although this experiment demonstrated that certain leafy indigenous vegetables could be grown in water-deficient conditions, it is crucial to water the plants at the seedling stage until they are mature enough to withstand extreme water stresses.
Water deficit limits edible biomass production in the leafy African vegetables of the study. The edible fresh weight decline in water-deficient varieties is directly related to the decrease in their nutritional values. At the content level, the values showed an increase in water-deficient varieties. Amaranth and African nightshade have shown promising results in drought conditions, Ethiopian Kale, however showed poor yields. Among each crop groups, A2 (A. hypochondriacus) and N1 (S. scabrum) are recommended as drought tolerant indigenous vegetables for community adaptation programs.
Acknowledgements
We acknowledge the collaboration between McGill University and AVRDC-The World Vegetable Center for the work on this study. We are grateful for Dr. Bernard Pelletier’s statistical support and the assistance of Jane Wu, the AVRDC Nutrition Unit, and Taiwan university students in the laboratory.
NOTES
*Corresponding author.