1. Introduction
A peptic ulcer, also known as PUD or peptic ulcer disease, is the most common ulcer of an area of the gastrointestinal tract. Peptic ulcers occur worldwide and gastric cancer is the second commonest cause of death from malignant disease [1]. It is defined as mucosal erosions equal to or greater than 0.5 cm. Almost all ulcers are associated with Helicobacter pylori, a spiral-shaped bacterium that lives in the acidic environment of the stomach. They are caused by many factors such as drugs, stress or alcohol, due to an imbalance between offensive acidpepsin secretion and defensive mucosal factors like mucin secretion and cell shedding [2].
A number of drugs including prostaglandins analogs, histamine receptor antagonists, proton pump inhibitors, and cytoprotective agents are available for the treatment of peptic ulcer. By the way various side effects of these products such as, hepatotoxicity and anaphylaxis, are not totally managed yet, so medicinal plant as an alternative treatment always has been the focus of many studies [3,4].
A long-term use of non-steroidal anti-inflammatory drugs (NSAIDs) causing inflammation of the gastric mucosa, gastrointestinal associated with a range of toxicity, and may finally cause ulceration, bleeding and changes with a very high morbidity and mortality [5- 7].
Currently due to several adverse effects of chemical drugs, herbal medicines are generally used in treatment. Several natural products have been reported to poses anti-ulcerogenic activity by virtue of their predominant effects on mucosal defensive factors including apple bananas, papeeta, and brindleberry [8,9].
Pomegranate (Punica granatum L.) is a well-known table fruit of tropical and subtropical regions of the world. Some botanists place it in the family Lythraceae, of the peculiar type of fruit, called as balausta, most authorities make it the only genus in the family Punicaceae. It belongs to genera Punica and family Punicaceae [10]. It is mainly grown in Mediterranean regions and is one of the major cultivated productions of Iran and also in Afghanistan [11,12].
The goal of many studies has been to identify the therapeutic constituents of pomegranate. Commonly found in many plants, ellagic acid exhibits powerful anticarcinogenic and antioxidant properties, propelling it to the forefront of pomegranate research. The pericarp part of pomegranate showed more antioxidant content including phenolic punicalagins; gallic acid and other fatty acids; catechin, quercetin, rutin; flavones, flavonones; anthocyanidins [13-15]. In addition to its ancient historical uses, pomegranate is used in several systems of medicine for a variety of ailments. It used as an antiparasitic agent, a “blood tonic” in addition to heal aphthae, diarrhea, and ulcers. Pomegranate also serves as a remedy for diabetes [15,16].
Hence the aim of the current study is to investigate whether pomegranate peel extract consumption has curative effect toward gastric ulcers and may be helpful in the prevention of indomethacin-induced ulcerogenesis.
2. Material and Method
2.1. Plant Material
There are many varieties of pomegranate in Iran, from very sour to very sweet in taste and from white, yellow, pink, red, purple and even black in color. Three fresh pomegranate cultivars were harvested randomly in September 2010 from different mature trees (14-year-old). The average temperature, the amount of rainfall, relative humidity, and the soil pH in growing season were 28.65˚C, 20 mm, 26% and 7.21, respectively. The trees were spaced 6 and 3 m between and along the rows, respectively. Trees were grown under traditional irrigation and routine cultural practices suitable for commercial fruit production. All cultivars were grown under the same geographical conditions and with the same applied agronomic practices. According to the list of Iranian pomegranate cultivars studied by Noormohammadi et al. [17] which studied on the Iranian pomegranate genotypes in two garden of Saveh germ plasm, the North white peel (Poost-Sefid-Shirin), Sour summer (TabestaniTorsh) and Black peel (PoostSiyahTorsh) are almost cultivated in Saveh (Markazi), and Ardakan (Yazd) respectively. Three cultivars of pomegranate including 1) North white peel, 2) Sour summer and 3) Black peel, were donated from Saveh Agricultural Investigation Center. Pomegranate fruits (three numbers of each cultivar) were collected and washed three times with distilled water.
The black pomegranates are almost rare and more expensive than others, since it is a natural medicine for various diseases. The North white peel, are the accessible cultivar in Iran and according to the Tarighi et al. [18], the north white peel mean length, width and thickness are 82.62 mm, 83.45 mm and 81.31 mm, respectively. Another prevalent pomegranate cultivar in Iran is Sour summer. The weight of each 100 seeds is 4.12 g and the ratio of its seed to its pulp is 55.
To prepare pomegranate extract, fresh fruits were peeled and 30 g of peels were weighted and extracted separately for 4 h by Soxhlet apparatus with methanol 80%. The extract of each cultivar were mixed and the extracts were concentrated under control reduced pressure at 40˚C to obtain the methanolic extracts.
2.2. Chemicals
All chemicals used in the experiments were of analytical grade. All reagents and solvents were purchased from Merck (Darmstadt, Germany) and Sigma (St. Louis, MO).
For laboratory experimentation, indomethacin, and cimetidine were obtained from Daroupakhsh Pharmaceutical Company (Tehran, Iran).
2.3. Pharmacological Experiments
2.3.1. Animals
Male Wistar rats weighing 175 - 220 g (Pasteur Institute, Tehran, Iran) were used in the study. The animals were fed under normal conditions (22˚C) in 9 separate groups consisting of 5 rats. Animal experiments were performed in accordance with national guidelines for the use and care of laboratory animals and approved by the local animal care committee of Tehran University of Medical Sciences.
2.3.2. Preparation of Test Samples for Bioassay
Test samples were administrated in traperitoneally (i.p.) after dissolving in saline (NaCl 0.9%). The control group animals received the same experimental handling as those of the test groups except that the extract treatment was replaced by administration of appropriate volumes of the dosing vehicle. The histamine-receptor type-2 (H2) blocker, cimetidine (100 mg/kg, i.p.), was used as a reference compound. Peptic ulcer induced by indomethacin. Test samples were given 1h before the i.p. administration of indomethacin [a non-steroidal anti-inflammatory drug] suspended in saline (50 mg/kg, 2 ml) to a group of five rats. Oral pretreatment with peel extracts (25, 50 and 100 mg/kg) for 15 days protected the gastric mucosa against the damage induced by indomethacin (50 mg/kg). Four hours later, the stomachs were removed and inflated with 10 ml of formalin solution and immersed in the same solution to fix the outer layer of stomach. Each stomach was then opened along the greater curvature, rinsed with tap water to remove gastric content and blood clots and examined under the dissecting microscope to assess the formation of ulcers [19].
The ulcer index (UI) and inhibition percentage was calculated according to previous reported [19].
2.4. Statistical Analyses
All data were analyzed by one-way ANOVA using SPSS version 16 (SPSS Inc) software. Differences among groups were obtained using the LSD option and Duncan test, and significance was declared at p < 0.05. The results were expressed as percentage and as mean ± standard deviation (mean ± SD).
3. Result and Discussion
Nowadays gastric ulcer is one of the most important concerns as a result of many factors especially widespread using of NSAIDs. Because of poorly understanding the pathophysiology of this disease [20], studies investigating new active compounds are needed. As well, various pharmaceutical products currently used for treatment of gastric ulcers are not completely efficient and cause many adverse side effects [3]. Consequently, it is necessary to develop more effective agents that are also less toxic, with medicinal plants being an attractive source for the development of new drugs because of their wide array of active ingredients [21].
In this study we used the model of indomethacin induced ulcer. As a matter of fact indomethacin is a NSAID and this class of drugs are widely used in clinical practice due to their efficacy and various therapeutic effects, on the other hand acute gastrointestinal lesions are the most serious and frequent side effects of NSAIDs, making them the most common cause of gastro duodenal ulcers in Western countries [22,23]. In present study indomethacin induced ulcerations in 100% of the rats. Rats in the control group suffered from very severe lesions, as shown in representative dissected stomach sections (Figure 1). The body weight of the rats was a homogenous parameter. There were no significant differences between rat groups.
Our data shows the anti-ulcer activity of methanol extract of pomegranate peel in experimentally indomethacin induced gastric ulcers in rats. The results of the initial trials carried out with different extracts (25, 50, 100 mg/kg) are presented in Table 1. Microscope views of dissected stomachs representing antiulcer effects of sour summer and in black peel extracts compared to indomethacine.
Statistical analysis showed a significant difference between the ulcer indexes between the cultivar (p-value < 0.05). From the Table 1, it is evident that 50 mg/kg dosage of sour summer cultivar markedly inhibited the peptic ulceras compared with indomethacin induced gastric ulcer. In comparison with positive control, mentioned dosage of sour summer inhibited indomethacin-induced
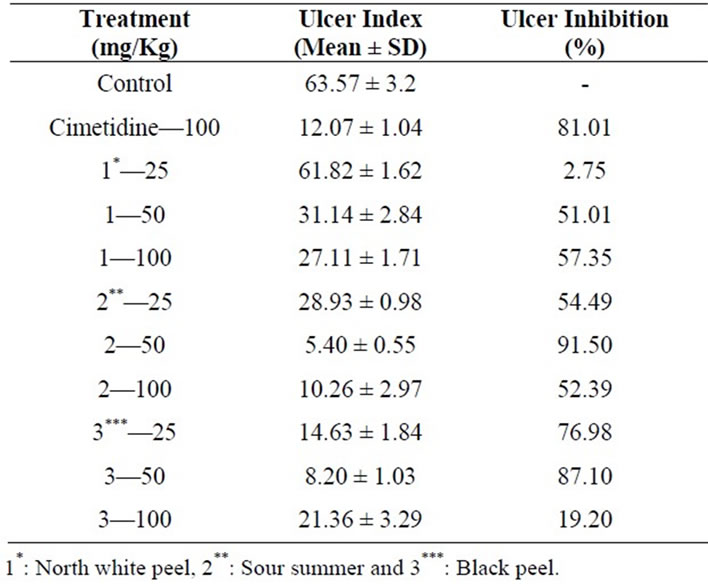
Table 1. Anti-ulcer effects of three different pomegranates peel methanolic extractions in rat.
peptic ulcer more potently than cimetidine. There was an apparent decrease in the infiltration of polymorphonuclear leukocytes and hemorrhage after administration of sour summer extracts (50 mg/kg) (Figure 1).
Using the sour summer and black peel cultivar with the dosage of 100 mg/kg decreased the level of ulcer index significantly (p < 0.05). Furthermore oral pretreatment with north white peel extract (25 mg/kg), significantly (p < 0.05) prevented the adverse changes and maintained the rats at near indomethacin induced status, while the group pretreated by the dosage of 50 and 100 mg/kg of same cultivar extract had a marginal with a little increase in ulcer inhibition. The submucosa was edematous and there was visible infiltration of polymorphonuclear leukocytes (Figure 1).
There are no related reports which describe antiulcer effect of pomegranate peel but in other studies fruit parts are assessed. Although the exact mechanism of the anti-ulcer activities of pomegranate peel has not been clearly delineated, it contains some active constituents which ulcer protective properties have been identified based on their antioxidant activity. Pomegranate is a rich source of polyphenols. It contains antioxidants like soluble polyphenols, tannins, and anthocyanins which possessing anti-atherosclerotic properties. The in vitro evaluation of the pomegranate extracts and some selected medicinal plants on H pylori activity demonstrate that the extract of pomegranate has remarkable anti-H. pylori function [24].
Alam et al. [25] claimed that in pylorus-ligated rats the ulcer lesion index, gastric volume, and total acidity significantly reduced by oral administration of an aqueous methanol extract of pomegranate fruit. It prevented ulceration by increasing the pH and mucus secretion in pylorus-ligated rats. Lai et al. [26] observed the antiulcer effects of pomegranate tannins in animal models. Pomegranate tannins play a protective role against gastric ulcer. It’s antiulcer effect is related to the increasing secretion of adherent mucus and free mucus from the stomach wall. This may inhibit generation of oxygen-derived free radicals, decrease the consumption of glutathione peroxidase and superoxide dismutase, and maintain the content of nitric oxide at a normal level [26].
In another research, the inhibition of gastric mucosal injury was evaluated by Ajaikumar et al. [27] Administration of 70% methanol extract of Punica granatum fruit rind revealed the gastro-protective activity of the extract through antioxidant mechanism. The in vivo antioxidant levels were increased and found in the normal values in treated groups of animals. All the histopathological examination of the stomach of the ulcerated animals which showed severe erosion of gastric mucosa, sub-mucosal edema and neutrophil infiltration were found to be normal in treated groups.
Based on Shams Ardekal et al. [12] and Sadeghi et al. [14] study, the antioxidant activity of sour summer as a suitable source for extraction and purification of phenolic and flavonoid compound was higher than north white peel and black peel. The results of present study which is in line with previous data, show that pomegranate peel extract of pomegranate specially sour summer possess good potential as an antiulcer agent too. Additionally, no adverse effects have been reported on consuming pomegranate and its constituents, since time immemorial, animal studies have failed to report any toxicities at doses conventionally used in the conventional system of medicine [28].
4. Conclusion
It is worthy to note that the antioxidant capacity of pomegranate peel extract is 10 times higher than the pulp extract. Also a large quantity of pomegranate peel could be easily collected from the pomegranate processing industries or from the waste products originating. This suggests that the most extracts of pomegranate peel are both anti-inflammatory and anti-ulcerogenic. Taken together, the results can provide an extra income and may contribute to have good nutritional values of this product.
5. Acknowledgments
This work was supported by the grant (No: 88-01-33- 8530) from the research council of Tehran University of Medical Sciences, Tehran, Iran.
NOTES